Reports: B4
48565-B4 Solvent Effects in Supercritical Fluids
We are in the developmental stages of defining how supercritical fluids such as xenon, ethane, carbon dioxide, and trifluoromethane can be used as tools to study solvent effects in photophysical, photochemical, and chemical phenomena. Operation above the critical temperature precludes the complication of phase change when the process of interest is studied as a function of pressure at constant temperature. We thereby vary the bulk-density-dependent properties such as dielectric constant, refractive index, and viscosity. We also vary the local solvent number density at the solute (current model systems include pyrazine and benzene) in a predictable way. In so doing, we study solvent effects without changing the solvent. Our method includes quantum chemical calculation and molecular dynamics simulation to define the system, and experiment to measure the behavior of the system.
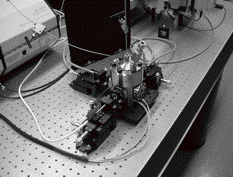
In this, the first year of PRF funding, we used these funds as summer salary support for two undergraduate students, Chaim Rube and Jonathan Schwab. We also used these funds and a generous match from the Yeshiva University Office of Academic Affairs to purchase and house major instrumentation, a combined steady-state and time-resolved spectrofluorometer. The time-resolved component of this instrumentation allows us to measure excited state lifetimes as short as 100 picoseconds. The light sources and the emission under supercritical conditions (see Figure 1) are coupled to the four-windowed, high-pressure, stainless-steel vessel via fiber optics and liquid light guides. This new instrument is housed in newly renovated laboratory space recently acquired by the PI. We are very close to completing all the preliminary work required for our first experiments with the solutes pyrazine and benzene in supercritical media. Our complementary absorbance work is ongoing in other laboratory space dedicated to this work.
Figure 1. High-pressure, stainless-steel vessel mounted on optical table, coupled with optics to a pulsed laser with continuous access to excitation wavelengths from 235 to 900 nm, a xenon arc lamp for steady-state excitation, and two photomultiplier tubes dedicated to the time-resolved or steady-state emission.
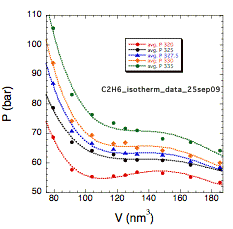
Determination of the critical temperature of an all-atom ethane model (322.2 K) represents our recent progress with the molecular dynamics component of our work. An undergraduate ran simulations to define isotherms close to the critical temperature for a system of 500 ethanes and one pyrazine according to a standard Lennard-Jones potential. The four isotherms (325, 327.5, 330, and 335 K) above the critical temperature were fitted with fourth-order polynomials (see Figure 2). Solutions to the first derivatives at the critical volume were plotted against temperature; the T intercept gives the critical temperature for these simulation conditions.
Figure 2. Isotherms for our Lennard-Jones simulation of all-atom ethane used to determine the critical temperature, 322.2 K, which is within about 5 percent of the experimental value, 305.3 K.
We are grateful for support from the Petroleum Research Foundation, and look forward to acknowledging this support in forthcoming publications.