Reports: AC6
46108-AC6 Ion Evaporation from Taylor Cones of High-Conductivity Electrolytes Immersed in Hydrocarbons
1. Charge injection into non-polar liquids from electrosprays of highly conducting liquids
In our previous report we studied electrosprays (ES) of the ionic liquid (IL) EMI-BF4 (EMI=ethylmethylimodazolium+) immersed in a quiescent heptane bath. We were (provisionally) disappointed to find that, on average, the charged particles injected were IL nanodrops rather than ions. We also noted that, when the heptane bath presents a free surface, the charged IL nanotrops injected into its bulk drift into this surface, and destabilize it forming an electrospray of heptane. We have now characterized thoroughly this compound electrospray, and find a simple scaling law for the size of the heptane drops:
D = G(e) (eogQ2/I2)1/3, with G(e) = 4.6 ± 0.25 for heptane (e~1.92), (1)
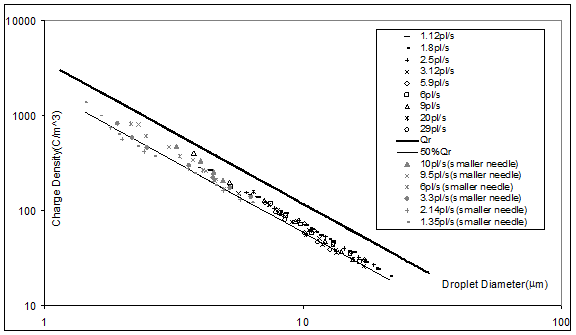
In this equation I/Q is the mean charge density measured (= spray current over heptane flow rate) for the emulsion of nanodrops, eo is the electrical permittivity of vacuum, e is the dielectric constant of heptane, and g is the surface tension of heptane/air. This result simply means that the heptane drops are charged to approximately 60% of the Rayleigh limit, as can be seen in Figure 1. This work is under review in Phys. Fluids, pending small improvements.
Figure 1. Measured current over flow rate of electrosprays of heptane versus measured drop diameter D when injecting an electrospray of EMI-BF4 within the heptane bulk. The drops are close to 60% of the Rayleigh limit, whence D can be predicted from readily measurable quantities,
A singular observation is that much larger currents can be injected in this coaxial electrospray than in quiescent heptane (150 nA vs, 15 nA). The apparent reason is that entrainment of the charged nanodrops by the fast heptane jet relieves the strong space charge effects limiting the current in the quiescent bath. Therefore, at decreasing Q the heptane jet becomes thinner and its speed increases, resulting in an increasing current and a further decrease of the jet size. We have exploited this effect by operating under conditions of unusually high current I and low heptane flow rate Q. We have as a result discovered the possibility of producing insulating drops as small as 250 nm! Even smaller sizes can apparently be produced, which we expect will lead to drastic advances in electrospraying of insulating liquids for analysis of insulators (such as petroleum products).
Because the lowest drop size achievable in heptane appeared to be limited by its volatility, we have recently extended the study of the high I/Q regime with decane sprays. We have as a result discovered conditions where the charge density increases above the Rayleigh limit for the observed drops. This is of course impossible, so it follows that under such stressed conditions a new mechanism for current production sets in. Our working hypothesis is that this new mechanism is direct ion injection from the IL into heptane, the very phenomenon we wished to investigate but had so far been elusive. There are other possible explanations of this finding, such as the production of many much smaller drops along with the maid drops observed, which would also be of great interest for electrospray mass spectrometry.
2. Ion mobility mass spectrometry studies
Two new research lines have been enabled by the recent availability of a unique combination of a differential mobility analyzer (DMA, which separates ions in space rather than in time) coupled to a mass spectrometer (MS) with a wide mass range (0-40 kDa). See Phys. Chem. Chem. Phys., 2009, 11, 8079-8090 for a description of its operation and capabilities. This facility has enabled (or will enable) the following studies
3. Composition of IL nanodrops and ions injected in insulating liquids
Our original plan was to analyze
the nanoparticles injected in the insulator by a DMA. However, the most
favorable (high I/Q) conditions discovered for ion injection involve a
heptane-air interface, so the ions and nanodrops to be analyzed will be in air
rather than heptane. We will soon start their analysis in the new DMA-MS
facility. The heptane drops will evaporate and release whatever ions and
nanodrops that have been produced by the
4. Electrospray ionization of non-polar substances
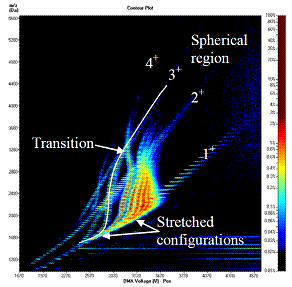
We have also studied the electrospraying of nonpolar substances dissolved in nonpolar solvents. As a first prototypical example we have sprayed polydimethylsiloxane (PDMS) in toluene/methanol (50/50 v). This mixture dissolves sufficient ammonium acetate to electrospray directly in air (conventional rather than compound ES). We confirm that at low charge states (high m/z) the polymer ions are spherical, greatly facilitating the determination of mass distributions via DMA-MS (Figure 2). This is similar as previously found for polar polymers such as polyethylene glycol (PEG), but now lower charge states are produced naturally.
Figure 2.- Separation of multiply charged polydimethylsiloxane ions as a function of inverse mobility (horizontal axis) and mass/charge (vertical axis), showing complex transitions from a crowded region of stretched configuration into a well separated region of spherical shapes