Reports: G9
45727-G9 Liquid Transport Mechanisms and Surface Interactions in Nanoporous Materials
Student research support
During this reporting period three undergraduates and one graduate student received professional development and technical training as a result of being supported grant. All four students were female, which are underrepresented in engineering. One of the undergraduates is a coauthor on the Journal of Membrane Science article supported in part by this ACS PRF grant.
Thermoporometric study of solvent-surface interactions
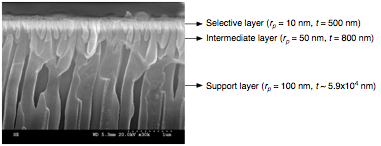
Year one, and the resulting manuscript, focused on examining and modeling solvent transport behavior through asymmetric nanoporous ceramic membranes as a function of membrane pore size and tortuosity. Subsequently, we have employed thermoporometry to gain insight into solvent-surface interactions, which can include van der Waals, oscillatory or ordering, hydrogen bonding, and electrostatic interactions. Anoporeª alumina membranes (Whatman) with 20 nm cylindrical pores were used as model membranes. Figure 1 shows a scanning electron micrograph of the membranes along with pore radii and thickness for each layer.
Figure 1. SEM micrograph of asymmetric alumina membranes.
To vary membrane surface chemistry, and thus these solvent-surface interactions, we used silane surface grafting with trichlorooctylsilane (C8H) and its fluorinated analog trichloroperfluoroalkylsilane (C8F). Results shown in Table 1 confirm silane grafting and a tri-bonding surface mechanism using Auger spectroscopy.
Table 1. Auger spectroscopy results for the silane modified alumina membranes. The native (unmodified) membrane yielded Al = 34.59 and O = 47.93.
|
Silane modifying agent
|
Elemental composition
|
|||||
|
Al
|
O
|
Si
|
C
|
Cl
|
F
|
|
|
trichlorooctylsilane (C8H)
|
20.45
|
37.96
|
9.06
|
29.73
|
0.55
|
|
|
trichloroperfluoroalkylsilane (C8F)
|
32.80
|
45.55
|
2.22
|
8.07
|
(–)a
|
1.36
|
Thermoporometry
is a differential scanning calorimetry (DSC) technique that measures melting
and freezing of a confined liquid in porous media. It is dependent on the
chemical composition of the media, the pore size and pore size distribution,
the pore geometry, and the surface chemistry. Changes in melting arise from
solvent-surface interactions and result in an unfreezable liquid layer of
thickness t on the membrane surface that
reduces the effective pore radius,
![]() . An effective pore radius can be calculated from an
expression that is analogous to the Gibbs-Thompson equation, which predicts
melting point depression in crystalline solids,
. An effective pore radius can be calculated from an
expression that is analogous to the Gibbs-Thompson equation, which predicts
melting point depression in crystalline solids,
![]()
(1)
where
![]() is the pore radius
of an unmodified membrane, t the
effective thickness of the SAM,
is the pore radius
of an unmodified membrane, t the
effective thickness of the SAM,
![]() the
solid-liquid interfacial tension,
the
solid-liquid interfacial tension,
![]() is the liquid
contact angle,
is the liquid
contact angle,
![]() the liquid
density,
the liquid
density,
![]() the fusion
enthalpy,
the fusion
enthalpy,
![]() is the
difference in melting temperature between the confined and bulk phases, and
is the
difference in melting temperature between the confined and bulk phases, and
![]() the liquid
bulk melting temperature. Parameters associated with the different probe
liquids and calculated (predicted) changes in melting temperature are shown in
Table 2.
the liquid
bulk melting temperature. Parameters associated with the different probe
liquids and calculated (predicted) changes in melting temperature are shown in
Table 2.
Table 2. Properties of the probe solvents used and calculated changes in melting between the confined and bulk phases in the selective, intermediate, and support membrane layers.
|
Probe solvent
|
|
|
|
|
|||
|
(oC)
|
(J g-1)
|
(mN m-1)
|
(g cm-3)
|
|
50 nm
|
100 nm
|
|
|
Decane
|
-29.7
|
201.7
|
13.5c
|
0.765
|
-2.1
|
-0.4
|
-0.2
|
|
Water
|
0
|
333.4
|
25.5
|
1.000
|
-2.1
|
-0.4
|
-0.2
|
a obtained from DIPPR database
b at liquid melting temperature
c based on dodecane
d equation (1)
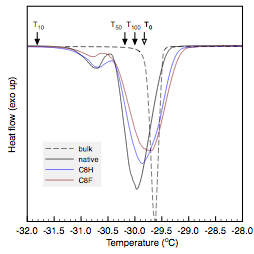
The melting temperature (onset) of water was observed at -0.8 oC for all samples, presumably due to nucleation at the DSC pan surface (Figure 1, top). Pore melting was observed for water in the native and C8H modified membranes as denoted by the small endotherm with an onset temperature of -1.4 oC. These results yield calculated effective pore radii of 34.7 nm, which is attributed to nanoscale confinement in the intermediate layer. Pore melting in the C8H membrane indicated that water was able to intrude into the membrane in spite of the hydrophobic modification. No water intrusion was observed in the C8F membrane, which is attributed to the greater surface hydrophobicity of the fluorinated pore surfaces.
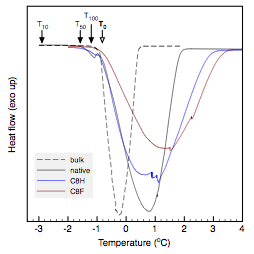
The melting temperature for pure
decane, denoted by the onset temperature
![]() , was -29.7 oC. In the presence of all
three membranes, pore and bulk melting was observed at onset temperatures of
-31.2 and -30.4 oC, respectively. Based on these temperature shifts,
effective pore radii of 14.2 and 30.4 nm were calculated based on complete pore
wetting (
, was -29.7 oC. In the presence of all
three membranes, pore and bulk melting was observed at onset temperatures of
-31.2 and -30.4 oC, respectively. Based on these temperature shifts,
effective pore radii of 14.2 and 30.4 nm were calculated based on complete pore
wetting (
![]() = 0,
equation 1). These pore radii are attributed to the selective and intermediate
layers, respectively. Decane can interact more favorably than water with the
hydrophobic surfaces. Comparing decane to water suggests that unfavorable
surface interactions influence melting under confinement.
= 0,
equation 1). These pore radii are attributed to the selective and intermediate
layers, respectively. Decane can interact more favorably than water with the
hydrophobic surfaces. Comparing decane to water suggests that unfavorable
surface interactions influence melting under confinement.
Figure 1. Thermoporometric scans of water (top) and decane (bottom) melting under nanoscale confinement in native and surface functionalized alumina membranes.
Role of solvent-surface interactions on separation performance
With knowledge of pure solvent transport and solvent-surface interactions, we are now exploring the separation of polystyrene (1k and 5k MW) from cyclohexane using native and surface functionalized 1 and 5 kDa titania (TiO2) membranes. We have recently completed designing a system capable of online measurements of polystyrene concentration and thus separation. Our goal now is to identify how surface chemistry and the underlying transport behavior influence separation performance.