Reports: AC9
47976-AC9 Liquid Crystalline Membranes for Olefin/Paraffin Separations
Introduction
In this work we are developing liquid crystal (LC) based membranes which have selectivity targeted for specific olefin/paraffin separations using structural regularity and specific molecular interactions to achieve the separation. The primary challenges being addressed include (1) the controlled fabrication of stable, defect-free membranes and characterization of the resulting liquid crystalline order, and (2) the characterization of transport properties and selectivities for gas pairs such as ethylene/ethane, butylene/butane and cyclohexane/benzene as a function of liquid crystalline order and molecular structure. Specific liquid crystalline materials will be employed, which contain chemical groups or moieties that match the molecular structure of one of the gas components in the binary mixture. The idea is to enhance solubility and diffusion of the targeted component by enhancing molecular interactions and the ability of the gas component to fit into the liquid crystalline structure.
Research Impact
1. Fabrication of Liquid Crystal Based Membranes
Supported LC Membranes: Membranes consisting of a porous support impregnated with an LC material have proven useful in the evaluation of LC transport properties in solution-based separations. LC embedded membranes are prepared by impregnating the porous cellulose nitrate membrane with an LC material, such as 4-cyano-4'-octylbiphenyl (8CB) via vacuum filtration. A 25% (w/v) LC chloroform solution is filtered through the porous membrane support in and 8CB LC molecules adsorb to the porous support. The resulting LC embedded membranes are immersed in deionized water overnight at room temperature to remove any excess LC remaining on the membrane surface and then dried under vacuum for 1 hour at 70ºC. Supported liquid membranes were fabricated using a cellulose nitrate porous support and n-(4-Methoxybenzylidene)-4-butylaniline (MBBA), 8CB, and 4-cyano-4'pentylbiphenyl (5CB) respectively. Polarized light microscopy has been used to ensure that the LC materials form ordered phases within the porous support. The supported LC membranes have proven unstable under applied pressures (as described below). As a result, we have begun exploring other measurement options, and the development of more stable supported LC membrane sandwiches and polymer-dispersed liquid crystal (PDLC) membranes.
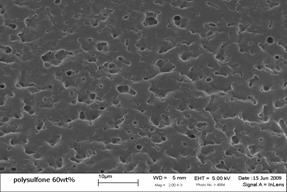
Polymer-dispersed Liquid Crystal (PDLC) Membranes: PDLC membranes are phase-separated structures consisting of pockets of the LC material dispersed in a polymer matrix. As a result, they are more stable to applied pressure than the supported LC membranes, but transport through the membranes is more difficult to model theoretically. In the supported membranes, the membrane transport can be treated as occurring entirely through the contiguous LC-filled pores, however, in the PDLC case diffusing molecules must pass both through the polymer matrix and the LC pockets. A Polysulfone/LC mixture is dissolved in chloroform (70 wt%) and sonicated at an elevated temperatures for 4 hours. The solution is cast on a glass substrate overnight while ensuring slow solvent evaporation. The resulting membranes are vacuum dried for several hours to remove any remaining solvent.
Membranes consisting of 40 wt% MBBA (above left) in Polysulfone lie above the solubility limit of the LC in the polymer (MBBA concentrations below 40 wt% showed no phase separation). This is shown by the formation of the domains that are presumed to be comprised of pure liquid crystal. The average domain size in these membranes is between two and four microns. Domain size is unaffected by liquid crystal concentration as variables such as temperature, evaporation rate, solvent, and sonication time are dominant, and remain constant between samples. At higher LC concentrations (60 wt% MBBA, above right) the LC droplets began aggregating due to the increase in domain concentration, resulting in larger cavities. A further increase in concentration should lead to the formation of separate macroscopic nematic and polymer domains which will lead to decreased membrane stability.
2. Transport Properties of Liquid Crystal Based Membranes
Supported LC Membranes: A constant volume/variable pressure apparatus was used for transport property measurement. Under testing, the liquid crystals separated from the support. This was due to the increase of pressure (1.5 2.0 atm) on the feed side, which overcomes the capillary forces keeping the LC material in the porous support. We have nearly completed a new permeation apparatus capable of measuring permeation at constant pressure and variable volume, allowing us to minimize the applied pressure. In this way, we will be able to characterize these less stable supported LC membranes.
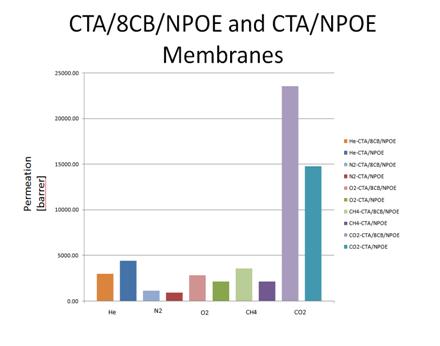
Polymer-dispersed Liquid Crystal (PDLC) Membranes: To date, only a small amount of data has been collected for inert gas transport through PDLC membranes, as depicted below. A comparison between solid CTA membranes and CTA/8CB membranes shows an increase in permeability in the PDLC membranes for all gases studied except helium. We do not have enough data to draw any significant conclusions from these results, but anticipate significant new data upon completion of the new permeation apparatus.
Career Impact:
The work has thus far provided support for one graduate student early in his PhD career. He has developed expertise in membrane fabrication and characterization (microscopy, SEM), transport property measurement, and has gained experience in the design, fabrication, and modification of laboratory instrumentation. In addition, the graduate student has been mentoring an undergraduate researcher, providing valuable lab experience for the undergraduate student and leadership experience for the graduate student. The project has had a significant impact on the career development of Dr. Martin, as it has allowed him to develop expertise and instrumentation in membrane characterization and to begin exploring the area of mixed matrix (PDLC) membranes.