Reports: G4
46549-G4 Structure-Function Studies of Indoleamine 2,3-Dioxygenase (IDO)
Fig. 2. Inhibition of IDO by ebselen.

Fig. 3. CD of IDO + ebselen (red) and ebselen (black).
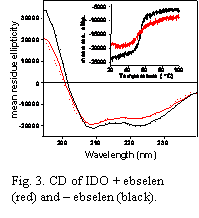
Progress this year has been made in two main areas of our research on the structure and function of Indoleamine 2,3-Dioxygenase (IDO). In the first, we have completed a detailed study of the mechanism of inhibition of IDO by the seleno-organic compound ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one; Fig. 1), which is known to display anti-inflammatory activity in vivo. This work was recently submitted to the ACS journal Biochemistry and is currently under review. In summary, we performed in vitro steady-state kinetic studies of IDO to determine the mode of inhibition of IDO by ebselen. The inhibition curve (Fig. 2) showed that ebselen is a very potent inhibitor of IDO with an inhibition constant of Ki ≈ 95 nM. Detailed modeling of the kinetics revealed that ebselen is a tight-binding inhibitor. We also performed equilibrium binding studies of the substrate L-Trp with IDO either treated or not treated with ebselen. These studies showed that ebselen treatment enhanced the binding of L-Trp to IDO (i.e., a positive cooperative effect on binding) despite being inhibitory to the reaction of L-Trp and O2 at the active site (data not shown). We interpreted these results as indicating that the reaction of ebselen with IDO causes the active site heme pocket to open up, allowing non-productive binding of L-Trp to the active site. We proposed that this was the principal cause of the enzyme inhibition. We supported this mechanistic hypothesis with a variety of spectroscopic data, including circular dichroism (CD), resonance Raman (RR) and UV-Vis absorption spectroscopy. The CD results showed that reaction of ebselen with IDO causes the protein to change its secondary structure and lose thermal stability (Fig. 3). RR spectroscopy showed that L-Trp and the diatomic heme ligand CO, which ordinarily interact tightly in the heme pocket, are pulled apart when ebselen reacts with the protein (Fig. 4). Together these results are consistent with ebselen reacting with IDO to cause a significant protein conformational change that leads to a significant opening up or restructuring of the heme active site. Finally, mass spectrometry results confirmed that ebselen reacts with cysteine residues of the protein to form selenosulfide covalent adducts (data not shown).
Fig. 4. RR of IDO-CO ± L-Trp ± ebselen.
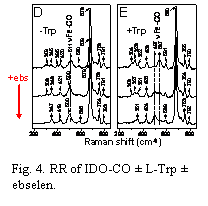
Fig. 5. Picture of PDMS Y-junction

Fig. 6. Confocal Raman image of myoglobin (purple) mixing with water (black) near the PDMS Y-junction (green).

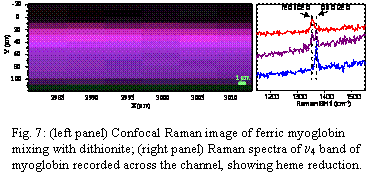
We also continued our work on the development of a rapid-mixing device for RR measurements of transient heme iron-oxo/Trp intermediates. Our work this year in this area was unfortunately hindered by the unavailability of our laser for more than six months due to a technical problem that needed repairing at great cost. The problem has now been fixed and progress on the project has been made. We have developed a microfluidic mixer based on a Y geometry with 100-200 mm wide by 50 mm deep channels. The channels are etched into PDMS polymer blocks that are fused onto glass substrates (Fig. 5). We tested the performance of the mixer first by mixing a myoglobin solution with a water solution and performing confocal resonance Raman microscopy scans across the mixing junction (Fig. 6). While mixing occurred we found it to be inadequate. For example, when ferric (oxidized) myoglobin solution was mixed with sodium dithionite solution (a strong reductant) we performed scans across the mixing channel to probe for reduced myoglobin (Fig. 7). As expected, a gradient of oxidized to reduced myoglobin was observed across the channel. The problem however is that reduced myoglobin was only observable in significant amounts a long way downstream from the Y-junction (in this case, 3000-5000 mm downstream). In other words, laminar flow prevails and the lateral mixing that occurs by molecular diffusion is too slow. We are currently in the process of testing mixers that have small vertical rods placed at the Y-junction to creat turbulent flow and hopefully rapid, efficient mixing.
Our research on IDO is revealing not only how this important enzyme performs its catalytic reactions but also how it may be regulated in vivo. The rapid mixing experiments combine leading edge microfluidic technology with the powerful spectroscopic technique of RR spectroscopy. There are only a few examples of this type of experiment currently in the literature. It is a novel approach that can reveal for the first time the transient intermediates involved in the oxidative reactions catalyzed by IDO. The technique can be applied to other enzymes as well. This research grant has enabled the P.I. to develop valuable new methodologies and technical capabilities that can be applied for years to come in the lab. The project has also provided valuable financial support and research experience for a talented, young Ph.D. student, Tito Sempertegui.
Fig. 7: (left panel) Confocal Raman image of ferric myoglobin mixing with dithionite; (right panel) Raman spectra of n4 band of myoglobin recorded across the channel, showing heme reduction.