Reports: G10
46584-G10 Inorganic Guest Species in Organic Crystals: A First-Principles Investigation
Overview and motivation
New organic electrode materials have the potential to dramatically expand the applicability of battery technology to new sectors such as automotive transport. Organic electrodes in rechargeable batteries must be able to intercalate ionic species that are either shuttled between electrodes or extracted from the electrolyte. The ability to design organic molecular building blocks with precisely defined electronic and steric features enables the synthesis of organic solids having electrochemical, kinetic and mechanical properties specially tuned for a specific battery application. However, before the rational design of such materials is possible, a fundamental understanding about the electronic, thermodynamic and kinetic properties of guest elements within various electronically conducting organic solids is essential. First-principles electronic structure methods can play a pivotal role in elucidating the mechanisms of charge transfer between electron donating or withdrawing guest species in electroactive organic crystals as well as rationalizing thermodynamic stability of and ionic mobility within organic electrode materials.
Accomplishments of the second year
During the first year of this Petroleum Research Grant, we devoted our efforts to developing an automated statistical mechanical software tool to predict phase diagrams and diffusion coefficients in intercalation compounds with arbitrary crystallographic complexity. This tool is essential to enable a systematic study of the thermodynamic and kinetic properties of different electronically conducting organic compounds. As a test case, we studied the finite-temperature thermodynamic properties of LixTiS2 as well as its concentration dependent Li diffusion coefficients.
Figure 1: Crystal structure of Di-lithium terephthalate.
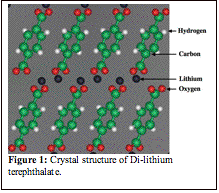
In the second year, we have been applying our statistical mechanical software infrastructure to study the electronic, thermodynamic and kinetic properties of Di-lithium terephthalate (Li2C8H4O4), a newly discovered organic electrode material for Li ion batteries. Di-lithium terephthalate can accommodate two additional Li ions at a constant voltage of around 1 Volt. The crystal structure of Li2C8H4O4 is illustrated in Figure 1. It undergoes a first-order phase transformation upon addition of Li. Our approach uses atomic-scale energies from first principles to parameterize an easily evaluated cluster expansion Hamiltonian, which can then be used in thermodynamic (Monte Carlo) and kinetic (kinetic Monte Carlo) simulations to determine diffusion constants. A Kubo-Green formalism is used to calculate diffusion coefficients for interstitial diffusion in the non-dilute regime with kinetic Monte Carlo simulations.
Since many electrode materials are synthesized to have nanometer dimensions, we have also devoted the second year to deriving thermodynamic equilibrium criteria for two-phase coexistence within nano-crystallites. As the electrode crystallites approach nanometer dimensions, the contribution of surface and interface free energies to the total free energy becomes sizable and cannot be neglected. The total free energy of a nano-crystallite in the simplest approximation can be written as a sum of bulk free energies plus surface and interface free energies multiplied by the surface and interface areas respectively. We have determined that the equilibrium criteria for two-phase coexistence in and among nano-crystallites differ from the well-known common tangent construction for large bulk phases. Equilibrium for two coexisting phases a and b within a single nano-crystallite for example must satisfy the following two criteria [3]
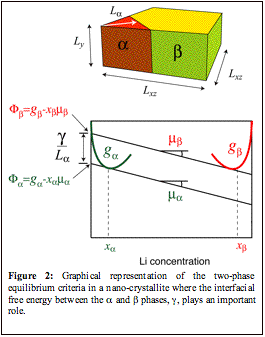
Figure 2: Graphical representation of the two-phase equilibrium criteria in a nano-crystallite where the interfacial free energy between the a and b phases, g, plays an important role.
 where the m
are the Li chemical potentials, the g
are the free energies, the x are
the Li concentration, the j
are the phase fractions, M is the number
of Li sites within the nano-crystallite, the Si are the surface areas of the different surfaces i and the Ak are the interface areas separating the a phase from the b phase. The Dsi are the differences in surface free energies between
the a and b phases while the gk are
interface free energies. Graphically, the above equilibrium criteria for a
diamond shaped nano-crystallite with a diagonal interface separating the a and b
phases correspond to the tangent construction illustrated in Figure 2.
where the m
are the Li chemical potentials, the g
are the free energies, the x are
the Li concentration, the j
are the phase fractions, M is the number
of Li sites within the nano-crystallite, the Si are the surface areas of the different surfaces i and the Ak are the interface areas separating the a phase from the b phase. The Dsi are the differences in surface free energies between
the a and b phases while the gk are
interface free energies. Graphically, the above equilibrium criteria for a
diamond shaped nano-crystallite with a diagonal interface separating the a and b
phases correspond to the tangent construction illustrated in Figure 2.
Most electrodes of Li-ion batteries in actuality consist of many nano-crystallites having a distribution of sizes. At the nanometer scale, thermodynamic properties such as the equilibrium voltage curve and compositions of two-phase coexistence depend on the size of the crystallite. We have also analyzed the effect of particle size distribution on the collective properties of a collection of nano-crystallites. The voltage curve of large electrode crystallites undergoing a two-phase reaction upon Li insertion exhibits a plateau as a function of Li composition. However, a collection of nano-crystallites having a distribution of sizes can exhibit a sloping voltage curve, similar to that of a solid solution, due to the fact that the plateau voltage depends on the particle size.




