Reports: AC1
45567-AC1 Rhodium-Catalyzed Addition of Alkynes to Activated Ketones and Aldehydes
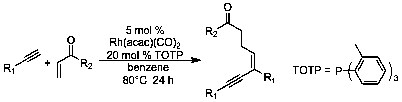
Multicomponent reactions are important processes for the synthesis of complex molecules. These reactions typically shorten synthetic routes to complex molecules by combining several reactions into a single step process, greatly improving efficiency. Recently during our studies on the rhodium catalyzed reactions of alkynes and electrophiles a new multicomponent process was discovered where two equivalents of the alkyne combined with an equivalent of a reactive ketone to provide an enyne of a single configuration (Eq. 1). The reaction is very sensitive to the phosphine used as a ligand for the rhodium, with tris(o-tolyl)phosphine providing the best yields (usually 60-80%, depending on the reaction components). Most alkynes participate well in the reaction, with the alkene coupling partner being limited to unsubstituted vinyl ketones and oxazolidinones.
Studies on the rhodium-catalyzed addition of alkynes to activated ketones and aldehydes are ongoing. Several chiral phosphine ligands were also prepared but only low enantioselectivities were observed. This is consistent with a mechanism where the phosphine ligand is not present in the coordination sphere of the metal complex during the alkyne addition. The synthesis of a chiral camphor derived rhodium complex was accomplished, but unfortunately, this ligand gave no enantiomeric excess in the alkyne addition reaction. Further studies on the development of improved b-diketone ligands are ongoing.