Reports: AC2
46557-AC2 Redox Reactions of Iron and Manganese that Chemically Transform Natural Organic Matter Within Sediments
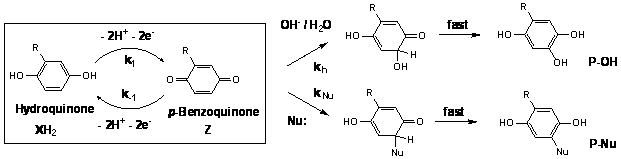
Dihydroxybenzenes (XH2) and benzoquinones (Z), believed to be the principal redox-active moieties within natural organic matter (NOM), are linked through redox reactions presented in the box below.
Although generally treated as reversible, one exception has been documented in the literature: Michael-Type bisulfide ion and mercaptan addition. In this reaction, benzoquinones serve as electrophiles and the RS- moieties serves as nucleophiles. Following nucleophilic attack, rapid tautomeric rearrangement yield substituted aromatic products, denoted above as P-Nu. This process contributes to the incorporation of sulfur into NOM during diagenesis within marine sediments.
Prior investigators focused on dihydroxybenzenes where the Hammett Constant of the ring substituent -R was either zero (for -H) or negative (e.g. for alkyl substituents.) It has generally been assumed that dihydroxybenzenes with electron-withdrawing substituents are resistant to oxidation, and hence unlikely to yield significant quantities of benzoquinones.
We have demonstrated that this assumption is incorrect. Our laboratory work indicates that manganese(III,IV) (hydr)oxides readily oxidize hydroquinones bearing positive Hammett Constant substituents, i.e. -Cl (sp = +0.24) -(C=O)OH (sp = +0.44), -(C=O)OCH3 (sp = +0.44), and -(C=O)CH3 (sp = +0.47). These electron-withdrawing substituents are frequently cited in structural characterizations of NOM, but up to now their effects on NOM reactivity have not been addressed. Manganese(III,IV) (hydr)oxides have been found at the oxic/anoxic interface within the water column of the Black Sea and within marine sediments.
We have hypothesized that electron-withdrawing groups markedly increase the electrophilicity of benzoquinones generated via reaction with manganese(III,IV) (hydr)oxides. Retardation of the oxidation step (e.g. a lowering of the k1/k-1 ratio) is more than offset by increases in kNu. Indeed, we have found that benzoquinones with electron-withdrawing substituents are so electrophilic that nucleophilic attack by OH-/H2O takes place; tautomeric rearrangement yields corresponding trihydroxybenzenes. Trihydroxybenzenes, in turn, are subject to oxidation, regenerating benzoquinones subject to a second adduct formation step. Overall, the original substrate can serve as a four-equivalent rather than two-equivalent reductant.
We hypothesized that increased electrophilicity brought about by electron-withdrawing substituents can facilitate Michael-Type adduct formation with non-sulfur nucleophiles, e.g. imidazoles, amines, phenols, oxyanions, and halides. In our first-year progress report, the use of liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) to document adduct generation was extensively described.
Our goal during the past year was to quantitatively compare reactivity among different benzoquinones and different nucleophiles. Our experiments began with p-benzoquinone. It is commercially available and readily purified by sublimation, hence oxidation by manganese(III,IV) (hydr)oxides is unnecessary. p-Benzoquinone is slowly attacked by OH-/H2O, yielding low amounts of trihydroxybenzene, which can be discerned by LC-ESI-MS. Adducts with nitrogen- and oxygen-donor nucleophiles can only be discerned when high nucleophile concentrations are employed. 500, 1000, and 1500 mM 4-methylimidazole, for example, yielded mono-adducts whose concentrations slowly increased during four hours of reaction. Chloro-p-benzoquinone, also commercially available, yielded similar results.
With gentisic acid, 2,5-dihydroxyterephthalic acid, 3,5-dihydroxyphthalic acid, and acetylhydroquinone, it is necessary to use MnO2(pyrolusite) to generate the corresponding benzoquinone. Fortunately, a number of geochemically interesting nucleophiles are resist oxidation by MnO2(pyrolusite) during the timescales of our experiments, e.g. 4-methylimidazole, 4-imidazoleacetic acid, 2-aminobenzyl alcohol, 4-chloroaniline, 4-methylaniline, 4-aminophenol, 4-nitrophenol, methyl 4-hydroxybenzoate, 4-hydroxyacetophenone, and 4-hydroxyphenylacetone. To initiate reaction, an aliquot of MnO2(pyrolusite) suspension was added to a buffer solution containing the substituted dihydroxybenzene and nucleophile of interest. In every instance, LC-ESI-MS yielded a peak with a molecular ion corresponding to the mono-adduct. For some combinations, di-adducts were also discerned.
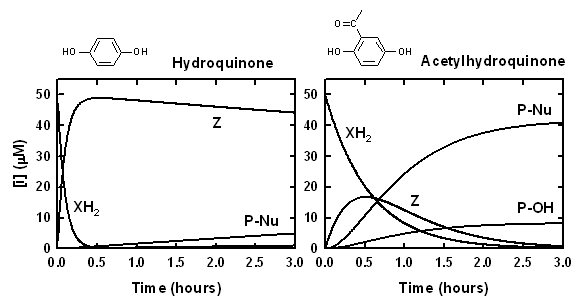
Two simulations, shown below, depict our expectations regarding dihydroxybenzenes lacking electron-withdrawing groups (left) and those possessing electron-withdrawing groups (right). Rate constants (k1, k-1, kh, kNu) have been arbitrarily selected:
The time course for Z is determined by the relative magnitude of source and sink terms. In the plot on the left, the p-benzoquinone concentration quickly reaches a value close to the amount of hydroquinone substrate that was added. Gradual conversion to the product P-Nu only occurs when a relatively high concentration of nucleophile (e.g. 4-methylimidazole) is employed. In the plot on the right, the acetyl substituent has an electron-withdrawing effect. As a consequence, formation of Z slows down, and at the same time adduct formation with added nucleophile and with OH-/H2O is accelerated. (These trends are even more pronounced with substrates bearing two electron-withdrawing substituents, i.e. 2,5-dihydroxyterephthalic acid and 3,5-dihydroxyphthalic acid.) The peak in concentration of Z is transitory, and relatively low. Formation of P-Nu and P-OH is governed by two parallel reactions. As long as the pH is fixed and an excess concentration of Nu: is employed, the yield of the two products should be fixed.
We are completing experiments where the concentration of added nucleophile (Nu:) is comparable to the anticipated amount of benzoquinone produced. The rate constant kNu is calculated using the initial slope of nucleophile versus time plots. We are also completing competitive nucleophile experiments. Adding 4-ethylaniline to suspensions containing 4-methylimidazole, for example, greatly diminishes the LC-ESI-MS peak corresponding to the 4-methylimidazole adduct. In this way, we have demonstrated that 4-ethylaniline is the more reactive nucleophile towards benzoquinones bearing electron-withdrawing groups.
We have performed a limited number of experiments employing iodide ion (I-) and 2-mercaptoethanol as nucleophiles. With these nucleophiles, it is necessary to first add the dihydroxybenzene substrate to the MnO2(pyrolusite) suspension to generate the benzoquinone, then remove unreacted MnO2(pyrolusite) by filtration prior to addition of the nucleophile. The time delay between generating the benzoquinone and adding the nucleophile makes it harder to quantify kNu.
We plan to complete the principal manuscripts based upon this work during the next six months. Ideas and information obtained through this work assisted in the completion of two additional works, which have been published (see citation report). Partial support of this work by the Petroleum Research Fund has been acknowledged.