Reports: AC4
47541-AC4 Photosensitizer-doped Organic Nanostructures and the Spatial Distribution of Singlet Oxygen
Summary
Micelles that contain photosensitizers can act as molecular-scale photoreactors with elevated internal concentrations of reactive intermediates. Such microheterogeneous systems are of interest because of their potential utility as water-compatible photocatalysts. This ongoing project is focused on measuring the internal concentrations of singlet oxygen, one such reactive intermediate formed in a microheterogeneous system. The nano-scale photoreactors in this study are comprised of well-defined block copolymer micelles that contain covalently and non-covalently bound sensitizers. The probe molecules trap singlet oxygen as high-energy dioxetane intermediates, which can be triggered to undergo chemiluminescent decomposition. Because the probe molecules are highly hydrophobic they partition into the core of the micelles and report the internal concentration of singlet oxygen. Localization of the probes inside the core is accomplished by covalent attachment to the block copolymer chains. Outcomes of this study include a new set of nano-scale photoreactor systems, development of a methodology for measuring intra-micelle singlet oxygen concentrations, and refinement of a quantitative kinetic model that predicts the distribution of singlet oxygen in microheterogeneous systems.
Introduction
The goal of this project is to measure the spatial distribution of singlet oxygen (1O2) generated in well-defined nanostructures that contain photosensitizers. Singlet oxygen, the first excited state of molecular oxygen, has a short lifetime (e.g., 4 ms in H2O) and thus a short diffusion length on the order of 100 nm (e.g., 99% quenching in 240 nm). When 1O2 is produced within a membrane, a micelle, a dendrimer, or some other macro- or supramolecular nanostructure, its short diffusion length leads to a steep concentration gradient with high internal and low external concentrations. In principle, such systems represent nano-scale photoreactors where photochemically driven reactions are considerably faster inside than outside. To predict which natural and synthetic systems make good photoreactors, a good understanding of the factors controlling the 1O2 distribution is needed; the crucial missing element is quantification of the local concentrations of 1O2. This problem cuts across numerous fields and arises whenever localized production of a short-lived, diffusible species occurs in a microheterogeneous system (e.g., photovoltaics).
Approach
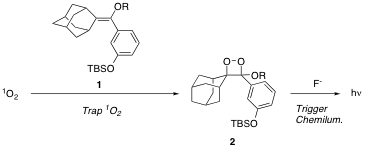
The approach for this project relies on two equally important recent advances. The first is the recent development in the McNeill lab of hydrophobic, sensitive, and selective probes for singlet oxygen. These probes work on the principle of dioxetane formation in the reaction of singlet oxygen with vinyl ethers, taking advantage of the chemiluminescent properties of dioxetanes for the detection step (Figure 1).
Figure 1.
The second important part of the approach is the utilization of block co-polymer micelles developed by Profs. Timothy Lodge and Marc Hillmyer that have well defined size and shape. The hydrophobic dioxetane precursor probe (1) selectively binds to the hydrophobic core of the block copolymer micelle. Singlet oxygen is generated photochemically using a hydrophobic dye (e.g., tetraphenylporphyrin), which also localizes in the micelle core. The probe reports the interior concentration of 1O2 and this can be compared the concentration measured in the bulk aqueous phase using conventional singlet oxygen probes such as furfuryl alcohol.
Results
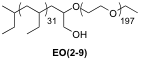
The microheterogeneous distribution of singlet oxygen in sensitizer-doped block copolymer solutions has been measured. In these experiments, an aqueous solution of EO(2-9) (Figure 2) was used. The model polymer is a poly(ethylethylene)-b-poly(ethylene oxide) [PEE-PEO] block copolymer with approximately 2 and 9 kDa hydrophobic (PEE) and hydrophilic (PEO) blocks, respectively. EO(2-9) is found to assemble into spherical micelles with a 7.5 nm radius hydrophobic core and a total radius of 24 nm. This was determined through a combination of cryo-transmission electron microscopy and dynamic light scattering To this solution, the water-insoluble sensitizer 5,10,15,20-tetrakis-(pentafluorophenyl)-21H,23H-porphyrin (FTPP) was added, which is taken up exclusively into the hydrophobic core. Measuring the microheterogeneous 1O2 distribution under irradiation with visible light showed higher concentrations of 1O2 in the hydrophobic regions than in the bulk solvent. The effect of enhanced interior concentrations is greater in this system was found to be three to four orders of magnitude higher than in the bulk aqueous solvent.
Figure 2.
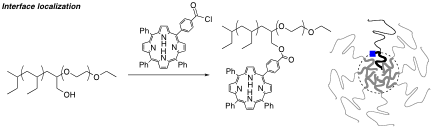
Additionally, a new class of block copolymer micelles have been prepared in which a sensitizer molecule is covalently linked at the junction of the two blocks (Figure 3). In this way, we have prepared micelles that have a sensitizer molecules localized at the hydrophobic-hydrophilic interface. We are currently exploring the photosensitizing properties of these new materials.
Figure 3. Left. Synthetic route to a diblock copolymer chain in which a tetraphenylporphyrin sensitizer is covalently bound at the junction between the blocks. Right. A schematic representation of a micelle with one sensitizer-functionalized chain and with the sensitizer at the hydrophobic/hydrophilic interface.