Reports: AC7
47774-AC7 Synthesis Characterization and Fundamental Studies of Novel Chiral Ionic Liquid and Their Polymers
Chiral ionic liquids type surfactants are of interest due to their ability to form micelles and lack of complete understanding of molecular recognition using these materials. In the first year of the PRF grant we have been mostly working along two major objectives: (a) synthesis of N-alkenoxycarbonyl-L-amino alcohols ionic liquid type surfactants with different chain length and head groups, (b) combined use of chiral ionic liquid and cyclodextrin for chiral separation of anionic and neutral compounds.
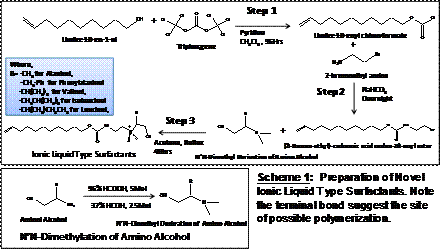
(a) Synthesis of N-alkenoxycarbonyl-L-amino alcohols ionic liquid (IL) type cationic surfactants with different head groups and chain lengths. Initial studies indicated that five cationic ILs with various head groups (alanine, valine, isoleucine, leucine, and phenylalanine) can be successfully synthesized as outlined in scheme 1.
Interestingly, L-alanine surfactant (L-UCAB) is a solid at room temperature whereas surfactant with L-valine (L-UCVB) head group is a semi-solid at room temperature. On the other hand, L-leucine (L-UCLB), which is more sterically hindered is a room temperature IL (RTIL) (Fig.1). In addition, two other ionic liquids with L-isoleucine and L-phenylalanine head groups are also RTILs. We have also synthesized leucine-based IL with three different chain lengths (butyl, octyl and undecyl). The most significant aspect of this study is the observation that the effects of head group and not the alkyl chain length controls the physical state of the synthesized cationic surfactants.
We are continuing to characterize the aforementioned surfactants using tensiometry, fluorometry and densitometry to determine the critical micelle concentration, aggregation number and phase ratio. Further studies are also planned in the second year of the project to develop a rational design for the synthesis of N-ureidocarbonyl-L-amino alcohol cationic IL surfactants and their polymers to understand the structure-physical state relationship.
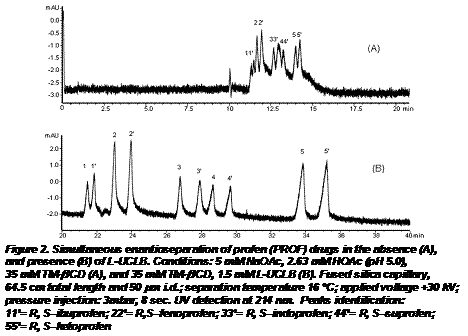
(b) Combined used of chiral ILs and CD for chiral separation of anionic and neutral compounds. We found that the presence of ionic liquid (e.g., L-UCLB) widen the scope of application of cyclodextrin (e.g.,trimethyl-b-CD, TM-b-CD) for chiral separations in capillary electrophoresis (CE). As shown in Fig.2A, TM-b-CD exhibits reasonable enantioselectivity for individual chiral separation of anionic profens, but it was less successfully in completely resolving the profen enantiomers in complex mixtures of structurally similar chiral profens. For example, only slight resolution was observed for ibuprofen enantiomers (1,1'). In addition, the later eluting enantiomers of indoprofen (3') co-eluted with the first eluting enantiomer of suprofen (4). Hence, TM-β-CD only partially separated the enantiomeric mixture of profens with a narrow chiral window. However, upon addition of only 1.5 mM L-UCLB to the CE buffer containing TM-β-CD, all enantiomers of profens were baseline resolved (except for ibuprofen, which provided Rs value of 1.32) with a significant widening of the chiral window (Fig.2B).
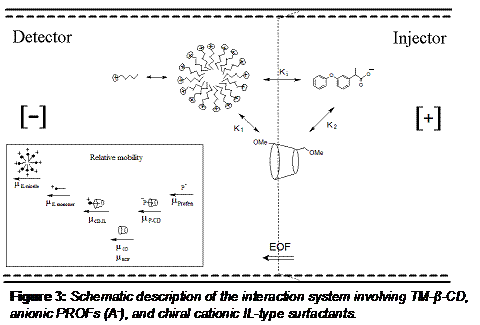
Furthermore, the peak efficiency improved by 3-10 folds (data not shown). These improvements in simultaneous enantioseparation of profens are due in large part to the interaction between TM-β-CD and L-UCLB, which in turn reduces the interaction of the L-UCLB with the capillary wall. We hypothesize that the IL, L-UCLB act as an inhibitor, reducing the interaction between TM-β-CD and the profens. To test the aforementioned hypothesis, we proposed a general model for the ternary interactions among profens, TM-β-CD and L-UCLB (Figure 3).
The concepts originated from enzyme kinetics were used to measure the binding constants of a ± profen drug using a mixture of IL and TM-β-CD as a dual chiral selector.
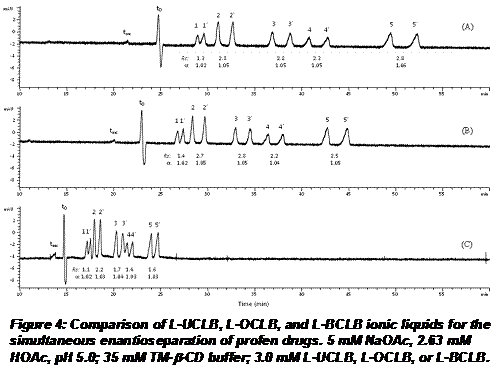
Effect of Chain Length: To study the effect of chain length, we compared three cationic ILs such as L-UCLB, L-OCLB and L-BCLB with eleven, eight and four carbon chain. Figure 4 compares the simultaneous enantioseparation of five profen drugs using the same concentration of the three cationic liquid surfactants. Note that the migration times of all profens and EOF are longer with the use of eleven carbon chain IL micelles than the use of eight and four carbon chain IL micelles. For example, the migration time of the latest peak (S-ketoprofen) eluted at 52.3 min when using L-UCLB. However, it eluted at 44.8 min when using L-OCLB, and only 24.7 min when using L-BCLB.
Unexpectedly, the chiral resolutions and selectivities of the racemic profen pairs did not change significantly when using L-UCLB and L-OCLB. The chiral resolutions of racemic profens were lower when using L-BCLB but the selectivities did not change significantly. This study suggests that L-UCLB, L-OCLB, and L-BCLB performed similar binding interactions with TM-β-CD. Moreover, the L-UCLB provided widest elution window compared to other two ILs. The measurement of elution window (Δt=|tmc-t0|) is 3.3 min for L-UCLB, 2.9 min for L-OCLB, and only 0.9 min for L-BCLB.
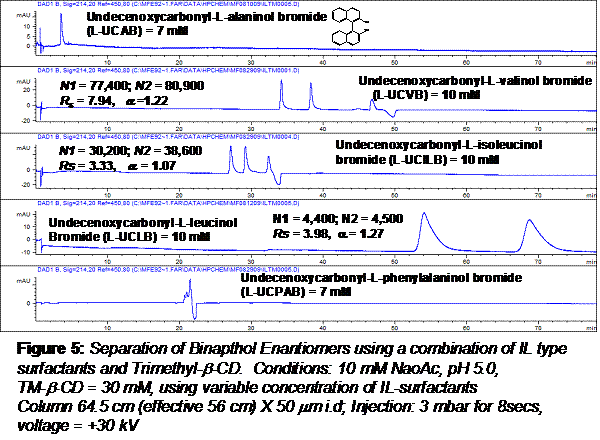
Effect of Head Group: The effect of surfactant head group on chiral separation of 1,1'-binaphthol is compared in Figure 5.
Several trends are evident. First, alanine surfactant which is a solid at room temperature provided no chiral selectivity (a = 1.00) whereas leucine based surfactant provided highest chiral selectivity (a = 1.27). Valinol surfactant seems to be the optimized IL because of higher efficiency, higher resolution but with very similar chiral selectivity as leucinol surfactant. Although no chiral selectivity was seen using phenylalaninol surfactant, we believe using higher or lower molar concentration of L-UCPAB will allow the two enantiomers to be separated from the neutral marker enabling enantioseparation.