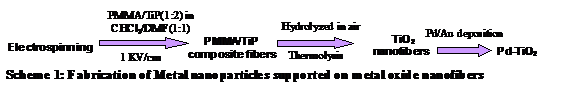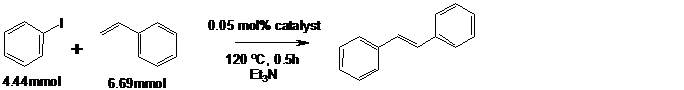Reports: AC5
46117-AC5 Nanofiber Catalyst Supports and Solution-Based Processes for Deposition of Catalytic Metals and Metal Oxides
The deposition of metal nanoparticles on molecular frameworks on a nanometer length scale such as in metal oxide or carbon nanofibers and nanotubes has been shown to enhance their catalytic properties due to high surface area to volume ratios, good charge transport properties, reduction in agglomeration and ease of separation from reaction mixture.1,2 We report the utilization of solution based bottom up' process in fabrication of these nanostructured materials. These materials were found to show high percent conversion, good selectivity, and higher yields at low catalyst loadings and high reaction temperatures.
In this final phase PRF report we report the successful deposition of Pd and Au nanoparticles on TiO2 nanofiber supports, scheme 1. These novel heterogeneous catalysts displayed enhanced catalytic activity for C-C coupling Heck reaction compared to commercially available catalysts supported on amorphous carbon. Complementary to this work, we also successfully fabricated metal and metal oxide nanotubes using a simple template based approach, where electrospun fibers served as a scaffold upon which a metal or metal oxide precursor was deposited via sol gel process,3 scheme 2. Copper sub-micrometer tubes were fabricated via electroless deposition on fiber template functionalized with polyaniline thin film achieved through in-situ polymerization. Pyrolysis of the polymer fiber template yield respective tubes.4
Figure 1. (a)Scanning
Electron Microscopy (
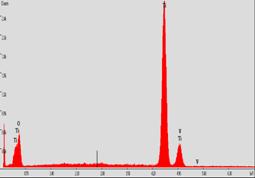
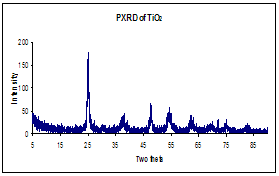
The morphology of fabricated nanocomposite fibers was smooth with dimensions ranging between 150±50 nm in diameter. Pyrolysis temperature, 500 °C, was based upon thermogravimetric analysis of the degradation profile of PMMA; the dimension averaged ~150 nm indicating complete removal of the PMMA. FTIR and Powder X-ray diffraction confirmed complete removal of organics leaving behind an anatase crystalline phase of titania. Pd and Au nanoparticles were uniformly distributed giving a 5 % loading with diameters ranging between 2 -10 nanometers over the TiO2 support.
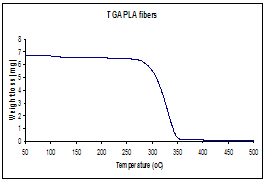
Figure 3. (a)Thermogravimetric analysis, TGA, illustrating the
thermal decomposition temperature of polylactide polymer, which determined the pyrolysis temperature of
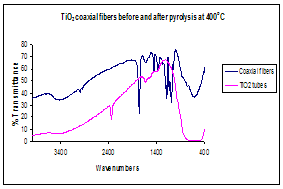
coaxial fiber (b) Infrared spectrum before and after calcinations indicating complete degradation of 
Figure 3.
The Pd-TiO2 catalyst was tested for Heck C-C coupling with iodobenzene, scheme (3). These reactions were carried out in an air atmosphere and comparisons were made with the commercially available Pd-C.
Scheme 3: C-C coupling of iodobenzene with styrene
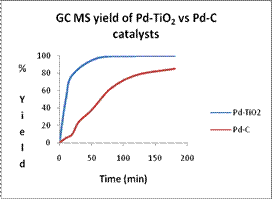
From the GC-MS yields, Pd-TiO2 gave a 99.6% yield in 30 minutes of reaction with no evidence of thermal degradation. These results suggest that the Pd-TiO2 catalyst is significantly better than the commercially available Pd-C catalyst which resulted in only an 85.0% yield with much lower conversion rate. Significant activity was reported for both bromobenzene (60% yield) and chlorobenzene which are relatively inactive with commercially available catalysts due to the increased bond strength.
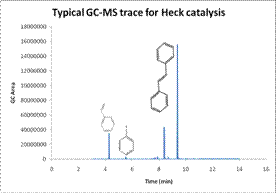
Figure 4. (a) A GC-MS trace for Heck reaction reactants and products from an aliquot. (b) Based on the recorded GC-MS areas on the left, the product % yield is obtained for Pd-TiO2 and compared with commercial Pd-C.
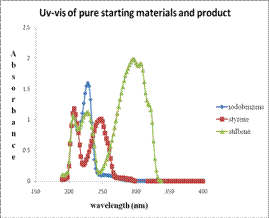
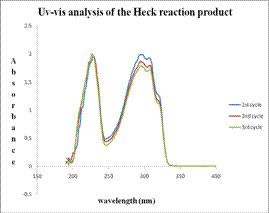
The major advantages of the Pd-TiO2 catalyst were the thermal and air stability coupled with high yields and short reaction times. This makes it an excellent candidate to replace the highly sensitive and difficult to handle homogenous catalysts (phosphine complexes) often used for more difficult transformations. Furthermore, the Pd-TiO2 catalyst was reusable and could be separated from the reaction mixture while retaining relatively high activity. This was demonstrated for example by Uv-vis analysis of the heck reaction under ambient conditions.
Figure 5. (a) Uv-vis spectra of pure starting materials and pure product of Heck reacton. (b) the Uv-vis spectra of starting material and product for three subsequent runs of Pd-TiO2 catalyst.
The heterogeneous nature of metal nanoparticles on metal oxide nanostructured materials provides for robustness, efficiency and complete elimination of residual heavy metals from the catalysts in the final product. This is crucial to the petroleum industry which relies on cheaper, robust and efficient catalytic systems which can adhere to Green chemistry', where limited waste-products are generated. Work in progress is geared towards mechanistic studies to understand the nature of very active metal species in solution. Similarly, related work is underway on fabrication and characterization of silver and copper nanoparticles and other metal oxide nanostructured supports such as ZnO and ZrO2 and their application to Heck and Suzuki C-C coupling reactions.
References
1. Dong, H.; Fey, E.; Gandelman, A.; Jones, W. E. Chemistry of Materials 2006, 18,
2008-2011.
2. Dong, H.; Nyame, V.; Macdiarmid, A. G.; Jones, W. E. Journal of Polymer Science
Part B-Polymer Physics 2004, 42, 3934-3942.
3. Ochanda, F., Cho, K.; Andala, D.; Keane, C. T.; Jones, W. E.,
Jr. Langmuir 2009
25,
7547.
4. Ochanda F., Jones W. E. Jr. Langmuir 2005, 21, 10791-10796.