Reports: G1
46850-G1 Development of New Methodologies Involving 2H-azirines
We have discovered that N-heterocycles can be synthesized through the functionalization of C–H bonds from the transition metal-catalyzed decomposition of readily accessible vinyl- and aryl azides. This report summarizes our research progress during the budget period 8/2008 – 8/2009 that culminated in the development of methods to access indolines, phenanthranils, and benzoisoxazoles. We also concluded a mechanism study of carbazole formation from aryl azides. These studies produced three papers that were published in Organic Letters and The Journal of Organic Chemistry.
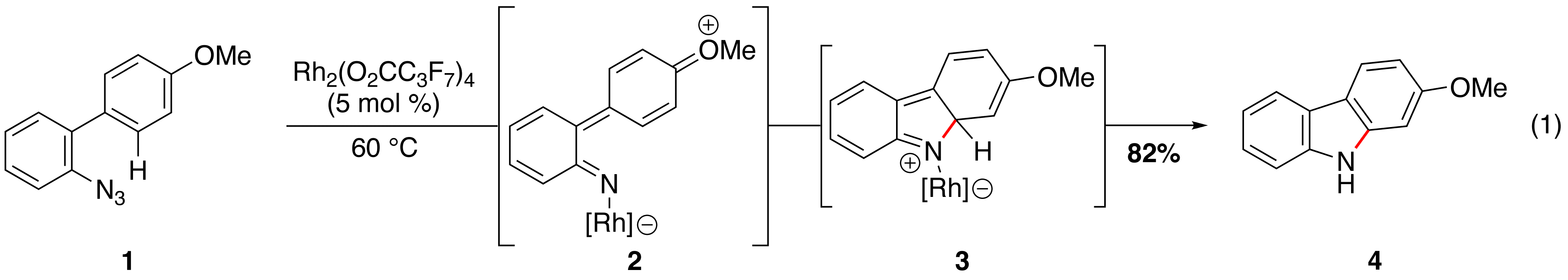
Rhodium(II)-Catalyzed Carbazole Formation. We completed our project to synthesize carbazoles from biaryl azides (eq 1, Publications 1 and 2). Intramolecular competition experiments revealed that electronic assistance was required to form rhodium nitrenoid 2. After nitrene formation, a 4¹-electron-5-atom-electrocyclization produced isocarbazole 3, which tautomerized to carbazole 4. These mechanistic experiments indicated that a contiguous ¹-system was required for rhodium nitrenoid generation from aryl azides.
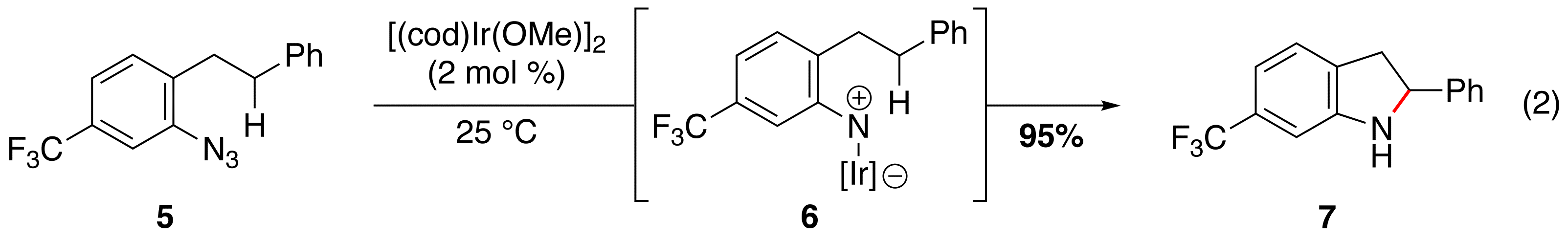
Ir(I)-Catalyzed Benzylic C–H Bond Functionalization. As a result of our mechanistic experiments, we examined a range of transition metals to achieve sp3 C–H bond functionalization. We discovered that [(cod)Ir(OMe)]2 (2 mol %) catalyzed the synthesis of indolines (e.g. 7) from electron deficient aryl azides (e.g. 5) at room temperature (eq 2, Publication 3). Our experimental evidence suggested that the functionalization of the benzylic C–H bond occurs through iridium nitrenoid 6.
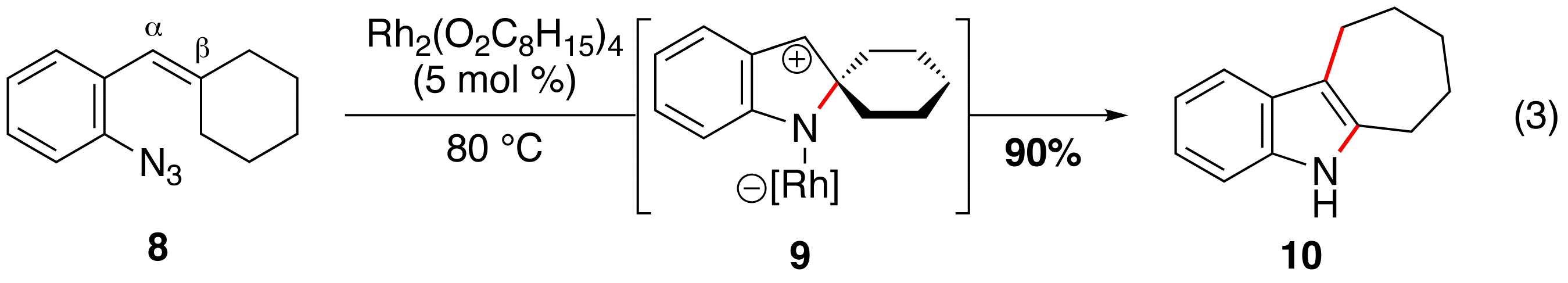
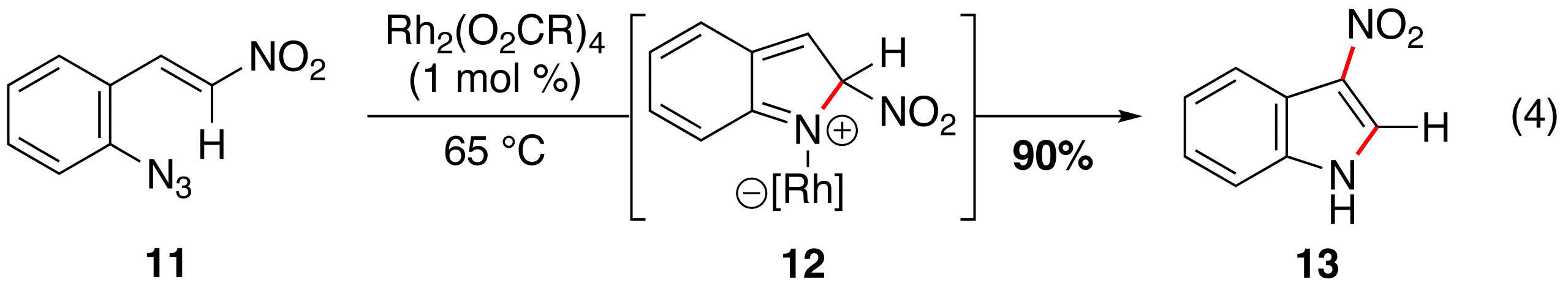
Rh(II)-Catalyzed Tandem Reactions. We discovered that migration of an alkyl group occured when aryl azides, which lack C–H bonds at the b-position (e.g. 8), are exposed to a rhodium(II) carboxylate (eq 3). This reactivity is not limited to b,b-disubstituted substrates: aryl azide 11, which contains a functionalizable C–H bond, produced 3-nitro-indole 13 as the only product (eq 4).
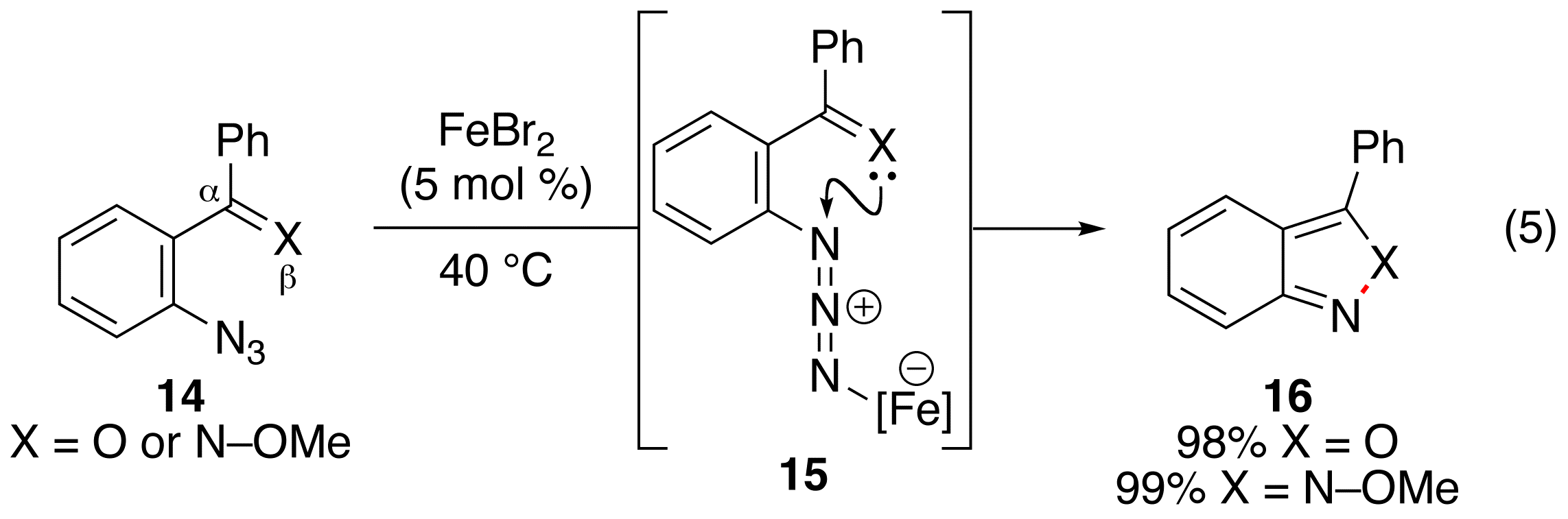
Fe(II)-Catalyzed N–O or N–N Bond Formation. We completed our examination of the relationship between the identity of the tether and the reactivity of the aryl azide (eq 5, Publication 4). We found that substitution of the b-C-atom with a heteroatom inhibited aryl azide decomposition by a rhodium(II) carboxylate. A screen of Lewis acids revealed that iron(II) bromide (5 mol %) promoted 2,1-benzisoxazole or 2H-indazole formation from the corresponding benzophenone or E-methyloxime 15 (eq 5).
In conclusion, we have found that transition metals can catalyze the transformation of aryl- and vinyl C–H bonds into C–N bonds using azides as the nitrogen atom source. The support of ACS-PRF enabled us to generate the requisite results to secure long(er) term funding from the National Institutes of Health to continue to study the reactivity of azides toward transition metals.
Publications.
(1) Stokes, B. J.; Jovanović, B.; Dong, H.; Richert, K. J.; Riell, R. D.; Driver, T. G. "Rh2(II)-Catalyzed Synthesis of Carbazoles from Biaryl Azides." J. Org. Chem. 2009, 74, 3225.
(2) Stokes, B. J.; Richert, K. J.; Driver, T. G. "Examination of the Mechanism of Rh2(II)-Catalyzed Carbazole Formation Using Intramolecular Competition Experiments." J. Org. Chem. 2009, 74, 6442.
(3) Sun, K.; Sachwani, R.; Richert, K. J.; Driver, T. G. "Intramolecular Ir(I)-Catalyzed Benzylic C–H Bond Amination of ortho-Substituted Aryl Azides." Org. Lett. 2009, 11, 3598.
(4) Pan, M.; Stokes, B. J.; Vogel, C. V.; Driver, T. G. "Intramolecular Fe(II)-Catalyzed N–N or N–O Bond Formation from Aryl Azides." manuscript submitted.