Reports: G3
44410-G3 Understanding the Origin of Suicide Inactivation in the Extradiol Dioxygenases
As described in the previous progress report, we have focused our efforts on the studies of the hydroquinone (HQ) ring-cleaving dioxygenases and Fe(II)-containing model complexes designed to mimic these enzymes. The results described below were used in an NSF-RUI grant that was submitted in July, 2009.
1. Studies of 2,6-dichlorohydroquinone 1,2-dioxygenase and related enzymes
Several Fe(II)-containing
ring-cleaving dioxygenases are similar to extradiol dioxygenases (EDOs), but operate
on very different substrates and are poorly characterized. One is 2,6-dichlorohydroquinone
1,2-dioxygenase from Sphingobium
chlorophenolicum (PcpA). Unlike the EDOs, where few enzymes can cleave
chlorinated substrates successfully, for PcpA, 2,6-dichlorohydroquinone
(2,6-diCl-HQ) is
 the native substrate.
the native substrate.
Determination of the active site of PcpA
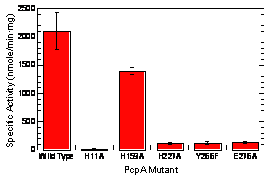
In an earlier progress report, we reported using a homology-based approach to construct a structural model of PcpA based upon a homologue of known structure, 1ZSW. We tested the location of the active site that was predicted by the structural model by site-directed mutagenesis. Two Whitman undergraduate students constructed mutants of PcpA in which each of the putative amino acids that could bind the iron (H11, H159, H227, and E276) was mutated to alanine. A fifth site-directed mutant was constructed in which a nearby amino acid that is conserved among all of these enzymes and is known to be critical for function in the EDOs was changed. The H11A, H227A, and E276A mutants exhibited less than 6% of the activity of the wild-type, thus showing that these are the residues that bind the Fe(II). In contrast, the H159A mutant retained about 67% of activity of the wild-type, which shows that H159 does not bind the Fe(II). The Y266F mutant showed 6% of the activity of the wild-type, showing that Y266 is also important for the function of the enzyme. The mutagenesis data confirmed our computer-generated structural model. A manuscript of this work was submitted to Journal of Biological Inorganic Chemistry and was accepted with minor revisions.
 We
are now attempting to obtain a crystal structure for PcpA. We sent protein to a
high-throughput crystallization facility and obtained several crystals. We are
now attempting to reproduce those results in-house in order to obtain crystals
of sufficient size for X-ray diffraction.
We
are now attempting to obtain a crystal structure for PcpA. We sent protein to a
high-throughput crystallization facility and obtained several crystals. We are
now attempting to reproduce those results in-house in order to obtain crystals
of sufficient size for X-ray diffraction.
Substrate specificity of PcpA
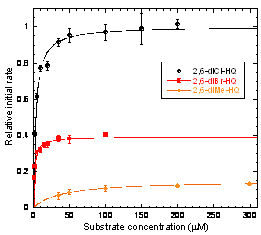
Another undergraduate student has performed steady-state kinetic experiments on PcpA. The native substrate, 2,6-diCl-HQ, has a Km of 3.±0.3 mM. 2,6-dibromo-HQ (2,6-diBr-HQ) and 2,6-dimethyl-HQ (2,6-diMe-HQ) are also substrates. The kcat and Km for 2,6-diBr-HQ are both somewhat lower than for 2,6-diCl-HQ, while for 2,6-diMe-HQ, kcat is ~7-fold lower and Km is ~13-fold higher. In contrast, 2-Cl-, 2-Br-, and 2-Me-HQ exhibit no evidence of ring cleavage, but are instead competitive inhibitors of PcpA. The KIs of monosubstituted HQs are in the following order: 2-Br-HQ < 2-Cl-HQ < 2-Me-HQ. HQ and 2,5-dichloro-HQ show no evidence of behaving has substrates or inhibitors.
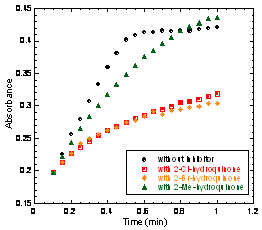
In addition, 2-Br- and 2-Cl-HQ also act as mechanism-based inactivators. When 2,6-diCl-HQ is used as the substrate, the plot of product formation versus time is strongly curved in the presence of either 2-Br- or 2-Cl-HQ. This curvature arises from progressive inactivation of the enzyme over time, and is known to occur in the EDOs for certain catechols that lead to mechanism-based inactivation. We are working to analyze these data for a clearer picture of this mechanism-based inactivation. Catechol, 3-methyl- and 4-methylcatechol, gentisate and pyrogallol are neither substrates nor inhibitors. 2,6-dichloro-, 2,6-dibromo-, and 4-amino-2,6-dichloro-phenol are weak inhibitors but do not show evidence of inactivating the enzyme.
 To
summarize: PcpA exhibits an absolute requirement for a hydroxyl groups at the
1- and 4-positions and for substituents at both the 2- and 6-positions in order
for ring-cleavage to occur. Halogens are greatly preferred at these positions.
Monosubstituted HQs are inhibitors and inactivators, and disubstituted phenols
are weak inhibitors. These observations have lead us to hypothesize that a weak
chloroarene-iron interaction could contribute to substrate binding and
specificity. We plan to test this hypothesis in several ways.
To
summarize: PcpA exhibits an absolute requirement for a hydroxyl groups at the
1- and 4-positions and for substituents at both the 2- and 6-positions in order
for ring-cleavage to occur. Halogens are greatly preferred at these positions.
Monosubstituted HQs are inhibitors and inactivators, and disubstituted phenols
are weak inhibitors. These observations have lead us to hypothesize that a weak
chloroarene-iron interaction could contribute to substrate binding and
specificity. We plan to test this hypothesis in several ways.
Progress on LinE
Another known hydroquinone dioxygenase, LinE from Sphingomonas paucimobilis UT26, has been reported to be active towards 2-chloro-HQ. In our last progress report we had cloned the LinE gene from Sphingobium indicum CCM 7286 (which is identical to that of S. paucimobilis UT26) and cloned it into a pET vector. This protein has proven to be difficult to express in a soluble form, but we remain hopeful that we will find a successful strategy. Our current approach is to co-express bacterial chaperone proteins.
2. Fe(II)-orthchlorophenolate model complexes
To probe the possible existence of
Fe(II)-haloarene interactions, we collaborated with Prof. Patrick Holland (
 The
1H NMR spectra showed the formation of the desired phenolate
complexes upon addition of several different substituted phenols as well as
2-Me-HQ to the Fe(II)(TACH-o-tol)
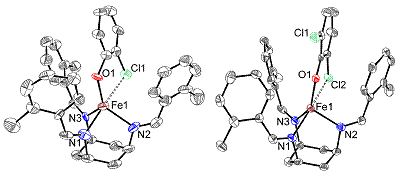
complex. Crystal structures were obtained for the [Fe(II)(TACH-o-tol)(2-chlorophenolate)]OTf
and [Fe(II)(TACH-o-tol)(2,6-dichloro-phenolate)]OTf complexes. These structures
show a distorted trigonal bipyramidal coordination geometry, in which the
chloro group and one of the nitrogens are in the axial positions, with Cl-Fe-N bond
angles of 168° and 167°/164° for the 2-chlorophenolate and
2,6-di-chlorophenolate complexes, respectively (the latter has two
nonequivalent molecules per unit cell). The Fe-Cl distances are 2.942 Å and
2.988/2.980 Å, respectively, for the two complexes. For comparison, the average
Fe(II)-chloride bond distances are 2.301 Å and 2.348 Å for
The
1H NMR spectra showed the formation of the desired phenolate
complexes upon addition of several different substituted phenols as well as
2-Me-HQ to the Fe(II)(TACH-o-tol)
complex. Crystal structures were obtained for the [Fe(II)(TACH-o-tol)(2-chlorophenolate)]OTf
and [Fe(II)(TACH-o-tol)(2,6-dichloro-phenolate)]OTf complexes. These structures
show a distorted trigonal bipyramidal coordination geometry, in which the
chloro group and one of the nitrogens are in the axial positions, with Cl-Fe-N bond
angles of 168° and 167°/164° for the 2-chlorophenolate and
2,6-di-chlorophenolate complexes, respectively (the latter has two
nonequivalent molecules per unit cell). The Fe-Cl distances are 2.942 Å and
2.988/2.980 Å, respectively, for the two complexes. For comparison, the average
Fe(II)-chloride bond distances are 2.301 Å and 2.348 Å for
 four-
and six-coordinate complexes, respectively, and the sum of the van der Waals
radii for Fe and Cl is 3.75 Å. These Fe-Cl distances are suggestive of
formation of an Fe(II)-Cl secondary bond. Very recently, the crystal structure
was obtained for the
four-
and six-coordinate complexes, respectively, and the sum of the van der Waals
radii for Fe and Cl is 3.75 Å. These Fe-Cl distances are suggestive of
formation of an Fe(II)-Cl secondary bond. Very recently, the crystal structure
was obtained for the




