Reports: AC9
45927-AC9 Measurement of Molecular and Thermal Diffusion Coeffcients in Model Petroleum Fluids
Diffusion of CO2 in water and in hydrocarbons in is increasingly recognized as an important part of transport of species in both improved oil recovery and CO2 sequestration. There are very little data at the conditions of the subsurface for both pressure and temperature. Theoretical modeling and predictions, especially for CO2-H2O mixtures, may not be also reliable.
There is only a handful of data
points for Fickian diffusion coefficients in
three-component mixtures in hydrocarbon mixtures. There is only one set of data
points for thermal diffusion coefficients in ternary mixtures to the best of
our knowledge. The main goal of the
project in the second year has been to employ the laser beam deflection
apparatus provided by Professor J. Sengers from the In the second year of the project after making various
effort to use the existing beam deflection apparatus we decided to design and
build a new diffusion cell to allow high accuracy in measurements. We also
developed a new model for calculation of Fickian diffusion coefficients in water-CO2
mixtures.
In the following, we will discuss the new cell design and
the results from it, and then move on to the theoretical work.
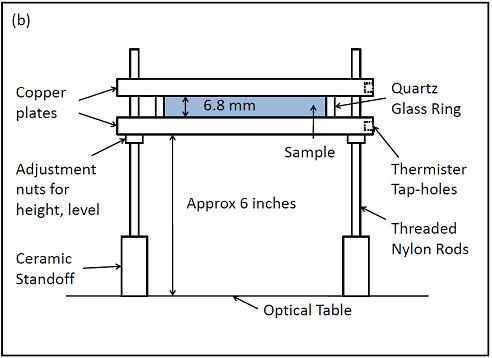
Figure 1 shows a schematic view of the new cell for the
laser beam deflection apparatus. The cell is composed of a quartz glass ring
sandwiched between two solid copper plates. The cell is leveled by adjusting
the nuts on which the cell sits. The spacing between the copper plates is 6.8
mm. Another design allows a spacing of about 3.0 mm. The later can reach stationary
state in time about 1/3 of the former.
Fig. 1- Schematics of the new laser beam cell.
Temperature control on the copper plates is achieved through
a PID system which controls the output of the power supplies, each one connected
to thermofoil heaters attached to the plates. There are two thermofoil heaters
attached to each plate. In order to prevent parasitic heating from erasing the
temperature gradient applied across the sample, the cell is placed in a cold
environment regulated by a thermostat. The temperature gradient in each plate
is around
The most important parameter which sets the accuracy and
reliability of diffusion measurements is the stability of the laser beam.
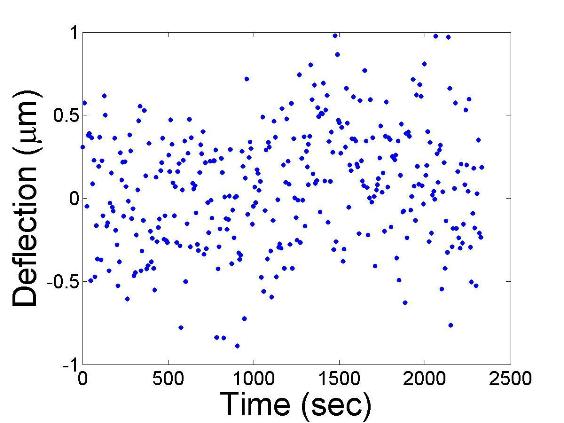
Figure 2 shows the fluctuations in the recorded stability of the laser beam at
a distance of about 50 cm from the outlet window.
Fig. 2- Fluctuations of the recorded laser beam at a
distance of 50 cm from the cell outlet.
The temperatures of the top and bottom plates were set at
298 K. The standard deviation of the fluctuations is around 0.35 microns. Such
low variations are superior to other measurements in the literature and allow diffusion
measurements, both Fickain and thermal diffusion coefficients, with high
accuracy. We have measured Fickian and
thermal diffusion coefficients of equimolar mixtures of hexane and toluene. The
results produce reported values in the literature. The stationary beam
deflection is around 0.215 mm from the time a stationary temperature gradient
is established. We are now in the process of measuring diffusion coefficients
of two similar normal alkanes nC9 and nC10
to examine the extreme accuracy of the new design.
The circular cell used in this work allows high pressure
modification once we complete atmospheric pressure measurements.
On the theoretical side, we have developed a model to
accurately calculate infinite dilution Fickian diffusion coefficients of CO2
in H2O, and of H2O in CO2 both
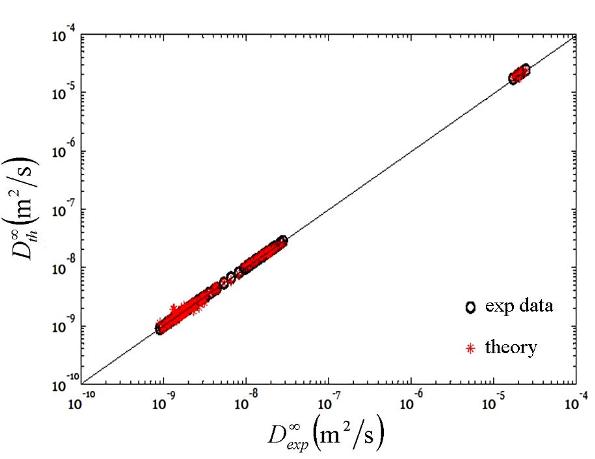
in the vapor phase and in the liquid state. Figure 3 compares our calculations
with measured data in the literature for the infinite dilution Fickian
diffusion coefficients. The data and the theory cover the vapor and liquid
states. Infinite dilution diffusion coefficients are required for the
prediction of concentrated mixtures. Our model allows calculations in vapor and
liquid states in a unified approach. Non-ideality in the vapor and liquid
states are accounted by a cubic equation of state combined a model for
association. We also account for cross association between water and CO2
molecules. In the process of model
development we have gathered all the data in the literature. The model is
expected to cover subsurface conditions considered for CO2 sequestration.
Fig. 3- Comparison of the computed and
measured infinite dilution diffusion coefficients of CO2-water
mixtures in vapor and liquid regions.
![]() 2 mK per
length of the plate. In our setup for a temperature gradient of about 1.6 K/cm
across the sample, the temperature on the top plate reaches to within 1% of the
desired set point. The temperature stabilizes in 3-4 minutes and fluctuates
about
2 mK per
length of the plate. In our setup for a temperature gradient of about 1.6 K/cm
across the sample, the temperature on the top plate reaches to within 1% of the
desired set point. The temperature stabilizes in 3-4 minutes and fluctuates
about
![]() 4 mK of the set point temperature of
the top plate. The lower plate's temperature is fixed.
4 mK of the set point temperature of
the top plate. The lower plate's temperature is fixed.