Reports: G3
45992-G3 Tunable Ligands for Rhodium-Catalyzed Hydroformylation
I. Final Project Synopsis and Structural Studies
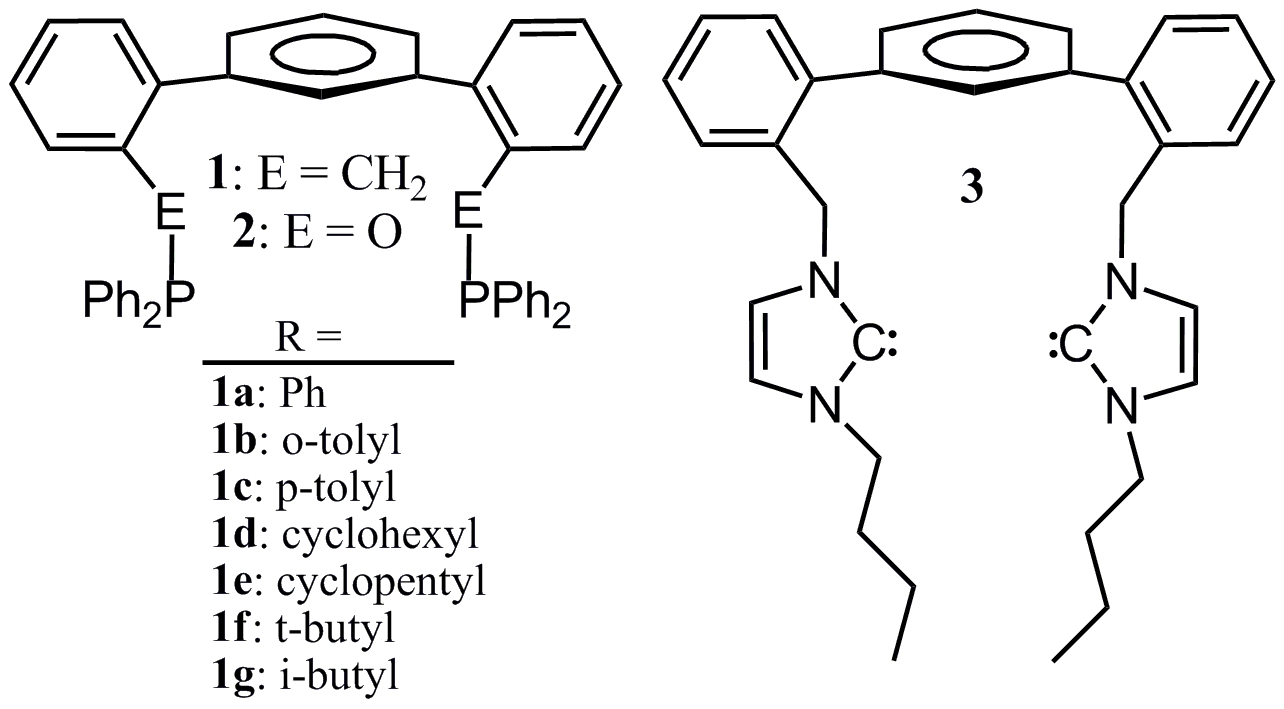
The main goal of this project was initially to examine the utility of a new class of phosphines (TERPHSPAN) in rhodium-catalized hydroformylation. Reviewer reports suggested that the study be expanded to include other transition metal-catalyzed reactions. The study of the TERPHSPAN scaffold was thus expanded to include Pd-catalyzed Suzuki coupling and Rh-catalyzed conjugate addition of aryl boronic acids to alpha, beta-unsaturated carbonyls. The ligand family was expanded to include seven diphosphines (1a-g), a diphosphinite (2), and a bis(N-heterocyclic carbene) (3). The structures of these ligands are shown in Figure 1.
Figure 1. Structures of Ligands
The coordination geometries of the ligands with Pd, Rh, Pt, and Ir were elucidated by single crystal X-ray diffraction, and representative structures demonstrating the flexibility of the ligands to accommodate different geometries as shown in the year 1 narrative report for this project. These structural studies were important data points for geometries for the initial TERPHSPAN ligand (1a). New TERPHSPAN diphosphines, in which phenyl was replaced with ethyl, isopropyl, n-butyl, i-butyl, t-butyl, cyclopentyl, cyclohexyl, o-tolyl, or p-tolyl groups, were prepared to elucidate the electronic and steric effects that such variation would have on on catalytic efficiency and regioselectivity.
II. Catalytic C-C Bond-Forming Catalysis utilizing TERPHSPAN ligands
We have probed three catalytic reactions using the original TERPHSPAN diphosphines: Rh-catalyzed conjugate addition of arylboronic acids to unsaturated carbonyls; Pd-catalyzed Suzuki-Miyaura coupling of arylboronic acids with aryl halides; and rhodium catalyzed hydroformylation of styrene and vinyl acetate. Hydroformylation results (Table 1) are very promising, with percent conversion and regioselectivity similar to that of commercially available Xantphos in some cases.
Table 1. Hydroformylation results
|
Ligand |
Time (h) |
Pressure (psi) |
Conversion (%) |
l:b ratio |
|
|
|
|
|
|
|
Ph |
3 |
200 |
6.95 |
1.64 |
|
Ph |
12 |
300 |
26.6 |
1.43 |
|
Ph |
12 |
200 |
26.8 |
1.18 |
|
Ph |
12 |
100 |
0.75 |
1.56 |
|
cHx |
12 |
200 |
19.27 |
1.45 |
|
oTol |
12 |
200 |
9.03 |
1.52 |
|
cPn |
12 |
200 |
36.4 |
0.74 |
|
tBu |
12 |
200 |
20.3 |
1.63 |
|
|
||||
|
Xantphos |
12 |
200 |
30.6 |
1.32 |
|
PPh3* |
12 |
200 |
27.3 |
0.96 |
|
|
|
|
|
|
|
Vinyl Acetate |
||||
|
Ph# |
12 |
200 |
2.15 |
0.27 |
|
Ph |
12 |
200 |
7.75 |
0.27 |
|
cHx |
12 |
200 |
13.0 |
0.18 |
All reactions were performed at 80 °C using 1:1 CO:H2 with L:Rh = 1.2 (1 M in toluene with respect to Rh) total substrate : Rh = 5000
* L : Rh = 2.4 because this is a monophosphine
# 50 °C was
reaction temperature
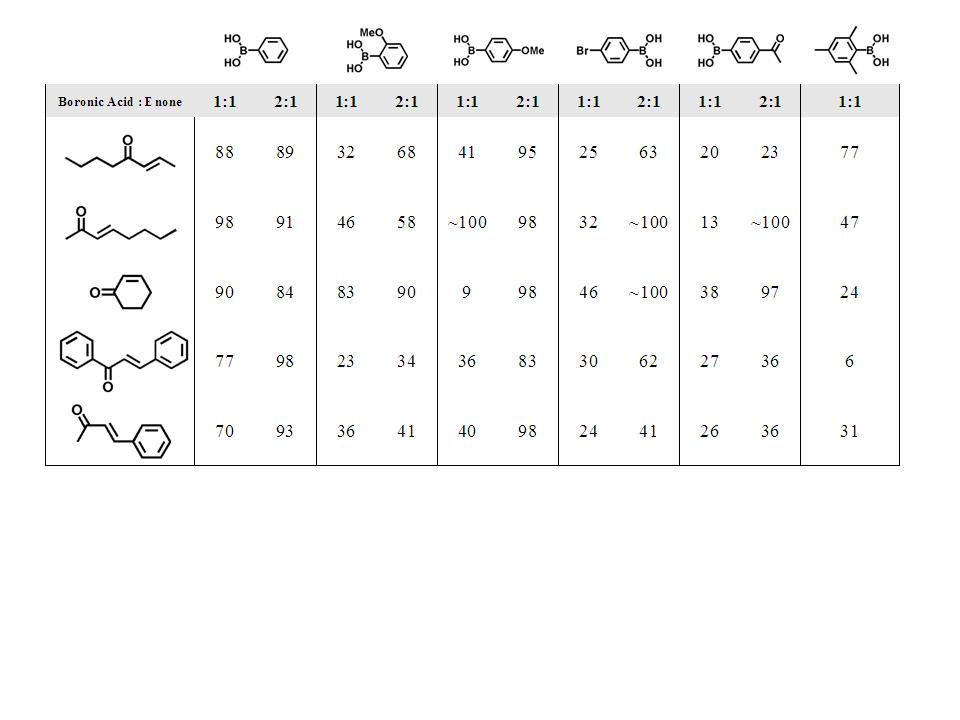
Conjugate addition reactions (Table 2)
also indicate
good activity, even for cases of sterically encumbered coupling
partners. Other
environmentally and economically noteworthy aspects of the conjugate
addition
reactions are that an organic / aqueous mixed solvent system was
employed, and
that the reaction is accomplished without using excess of the aryl
boronic acid
as is necessary when many other catalysts are used.
Table 2. Conjugate addition results
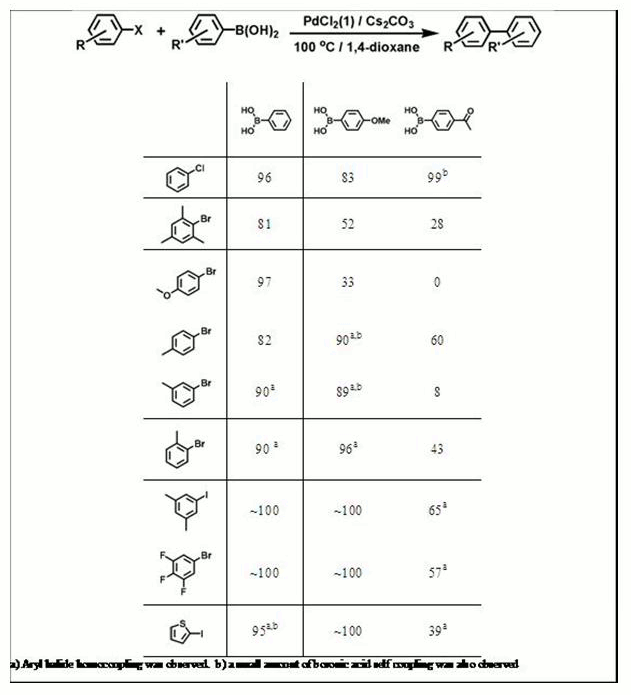
 Finally, for Suzuki coupling reactions,
TERPHSPAN-supported diphosphine 1a, diphosphinite 2, and bis(carbene) 3
were
compared. Complexes of 2 were found to decompose under the reaction
conditions,
while both 1a and 3 gave high yields and were capable of coupling a
variety of
aryl halides and boronic acids (Table 3).
Finally, for Suzuki coupling reactions,
TERPHSPAN-supported diphosphine 1a, diphosphinite 2, and bis(carbene) 3
were
compared. Complexes of 2 were found to decompose under the reaction
conditions,
while both 1a and 3 gave high yields and were capable of coupling a
variety of
aryl halides and boronic acids (Table 3).
Table 3. Suzuki coupling results