Reports: B10
47343-B10 Design and Synthesis of Novel Pyrene Discotics and Their Investigation in Organic Photovoltaic Cells
The design scheme of a typical discotic liquid crystal (DLC) material generally entails a central aromatic core embellished with three to eight flexible chains. Of great interest is the class of organic semiconducting DLCs as this class of materials has exciting electronic and optoelectronic properties. DLCs may form efficient π-π columnar stacks, which permit the achievement of high charge-carrier mobilities, whose magnitude is fundamentally determined by the degree of order and π-π molecular orbital overlap within the columnar stacks. The charge transport within the stack, i.e. in a one-dimensional manner, is therefore a built-in feature of the design scheme. Ease of film formation is another key property of smart materials for organic-based electronic and optoelectronic devices. As DLCs have a predisposition to self-organize into columnar stacks, an innate ease of processability is pre‑programmed.
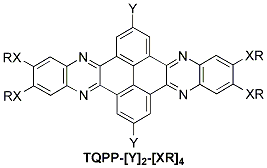
The synthesis of new materials was undertaken under the premise to use these materials in liquid crystalline photovoltaic applications. Discoid compounds 6,7,15,16-tetrakis(alkoxy/alkythio)quinoxalino[2′,3′:9,10]phenanthro[4,5-abc]phenazine (referred to as TQPP-[Y]2-[XR]4 (Y= H, t-Bu; X = O, S, R = CnH2n+1), Figure 1, were efficiently synthesized and characterized in our labs.
Figure 1. Chemical Structure of TQPP-[Y]2-[XR]4.
To evaluate the materials' suitability, a ménage of thermal and spectroscopic techniques was applied to study the phase structures and transitions of TQPP-[SCnH2n+1]4, namely differential scanning calorimetry (DSC), polarized light microscopy (PLM), wide-angle X-ray diffraction (WAXD), selected area electron diffraction (SAED), and Fourier transform infrared spectroscopy (FT-IR). When studying TQPP-[SC12H25]4 as a model compound using DSC and 1D WAXD, four crystalline and no liquid-crystalline phases were identified. The 2D WAXD experiments on oriented samples of TQPP-[SC12H25]4 and SAED patterns from single crystals revealed the unit cell of the lowest temperature TQPP-[SC12H25]4 crystalline phase (K1) to be monoclinic with dimensions of a = 1.87 nm, b =0.53 nm, c = 3.51 nm, and b = 96.2°. A controlled increase of the temperature, allowed the K1 phase to be transformed to the other crystalline phases which all were monoclinic with different crystallographic parameters. The various crystalline phases deviated only slightly in the placement of the aromatic TQPP-[SC12H25]4 core, yet strongly in the conformation of the alkyl chains attached to the rigid cores. Other TQPP-[SCnH2n+1]4 compounds possessed only two crystalline phases wherein the transition temperature can be fine-tuned by modifying the attached alkyl chains to the four corners of the rigid fused rings. Using one-dimensional WAXD studies, solely crystalcrystal transitions for the phase transitions of these compounds were identified. While single crystals of semiconducting materials would provide the highest charge carrier mobility, process costs skyrocket when considering the difficulty to grow single crystals while achieving uniform and large area coverage. The achievement of liquid crystalline phases in these compounds for their application as organic photovoltaic materials hence mandates the modifications to the alkyl chains. We are therefore currently exploiting the synthesis of TQPP-[t-Bu]2-[SPhytanyl]4. In addition, the mesophase behavior studies of TQPP-[t-Bu]2-[XR]4 (X = O, S, R = CnH2n+1) are currently ongoing.
Apart from molecular packing,
morphology, and phase behavior, optical and electronic properties such as
energy gap and a thorough understanding of light-triggered processes are of
vital importance for OPV materials. However, detailed photophysical
and photochemical study of discoid compounds remain scarce. The change of the
band gap, for example, can be significantly altered synthetically by
extension/shortening of the conjugation length and a minimization of
bond-length alternation along the conjugation length. We have undertaken an
in-depth spectroscopic characterization of TQPP-[t-Bu]2-[XCnH2n+1]4
(X = O, S). When compared to pyrene, TQPP materials
comprise a larger, conjugated core size, yet exhibit a nearly superimposible fluorescence behavior, also in terms of
monomer and excimer emission. Despite the observed
low solubility for TQPP materials, the solvatochromic
behavior of these materials was investigated in a series of solvents from polar
to nonpolar, i.e. methanol to cyclohexane.
Important photophysical properties such as Stokes
shifts, fluorescence lifetimes, fluorescence quantum yields, as well as radiative and nonradiative rate
constants were determined for a series of these TQPP materials in various
solvents, Table 1. The number of carbons in the alkyl chain R
for TQPP-[t-Bu]2-[OR]4
and TQPP-[t-Bu]2-[SR]4, correlated with the Stokes shift,
fluorescence quantum yield, and lifetime. We found a solvent-dependent quantum
yield in these compounds and a correlation of monomer to excimer
intensity ratio versus gross solvent scale (ET30)
and orientation polarizabilty (Δf).
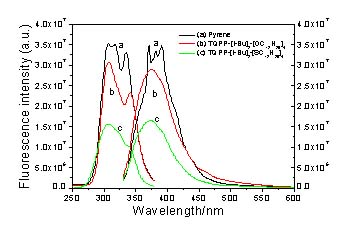
Furthermore, while TQPP-[t-Bu]2-[XR]4
showed a similar fluorescence and excitation behavior as that of pyrene (Figure 2), a relatively smaller lifetime was
observed for these compounds compared to that of pyrene.
Table 1: Monomer lifetime, excimer lifetime, fluorescence quantum yield, radiative and non-radiative rate
constants of TQPP-[t-Bu]2-[XR]4
in various solvents at 25 °C.
Compound
Solvents
τMonomer /nsec
τExcimer /nsec
ff
kr/108 s-1
knr/ 108 s-1
TQPP-[t-Bu]2-[OC12H25]4
Cyclohexane
2.59
11.50
0.79
3.03
0.83
1,4-Dioxane
3.29
15.80
0.87
2.64
0.40
1,2-Dichlorobenzene
1.91
12.08
0.69
3.63
1.61
THF
3.65
11.27
0.09
0.25
2.49
Dichloromethane
2.27
11.52
0.15
0.67
3.73
Methanol
2.58
9.91
0.20
0.78
3.10
TQPP-[t-Bu]2-[SC12H25]4
Cyclohexane
2.72
11.76
0.44
1.63
2.05
1,4-Dioxane
3.15
17.43
0.42
1.35
1.83
1,2-Dichlorobenzene
1.79
13.48
0.42
2.35
3.23
THF
3.99
12.34
0.15
0.37
2.14
Dichloromethane
2.77
11.49
0.09
0.34
3.27
Methanol
3.49
10.77
0.18
0.51
2.35
TQPP-[t-Bu]2-[OC10H21]4
Cyclohexane
3.41
12.20
0.88
2.58
0.35
1,4-Dioxane
5.02
19.10
0.67
1.32
0.67
1,2-Dichlorobenzene
2.28
13.32
0.17
0.75
3.64
THF
4.17
12.83
0.02
0.04
2.35
Dichloromethane
3.35
12.06
0.47
1.40
1.58
Methanol
3.56
10.57
0.83
2.32
0.49
TQPP-[t-Bu]2-[SC10H21]4
Cyclohexane
2.65
12.15
0.42
1.60
2.18
1,4-Dioxane
3.90
17.74
0.64
1.63
0.93
1,2-Dichlorobenzene
2.36
13.34
0.15
0.65
3.59
THF
4.85
11.95
0.09
0.19
1.88
Dichloromethane
3.10
12.10
0.17
0.53
2.69
Methanol
3.42
10.45
0.46
1.35
1.58
Figure
2. Excitation and emission
spectra comparison for pyrene, TQPP-[t-Bu]2-[OC12H25]4 and
TQPP-[t-Bu]2-[SC12H25]4 in
THF.