Reports: AC9
46419-AC9 Mechanistic Understanding of Wettability Alteration in Fractured Carbonate Reservoirs
The goal of this proposal is to develop a mechanistic understanding of the effect of potential determining ions, surfactants, oil composition, and temperature on the wettability of carbonate reservoir minerals. This knowledge can be used to alter wettability of initially oil-wet fractured carbonate reservoirs to recover oil from the matrix blocks. The effect of temperature on wettability and core-scale imbibition studies are reported here. Higher temperature effects and simulation of the imbibition process would be studied in the next year.
Wettability alteration of a calcite plate can depend on parameters like brine salinity, oil composition, type of surfactant, concentration of surfactant, and temperature. Most of these experiments are done with a model oil (1.5 wt% cyclohexanepentanoic acid in n-decane). Adsorption and desorption of crude oil components (e.g., naphthenic and carboxylic acids) alter the wettability of mineral surfaces. Temperature can have an effect on both oil/water and oil/water/mineral interfaces. The adsorption of naphthenic acids and other carboxylic acids is believed to be a chemisorption type of reaction affected by temperature. Also, the number of active Ca2+ sites on the solid surface is reduced at the higher temperature.
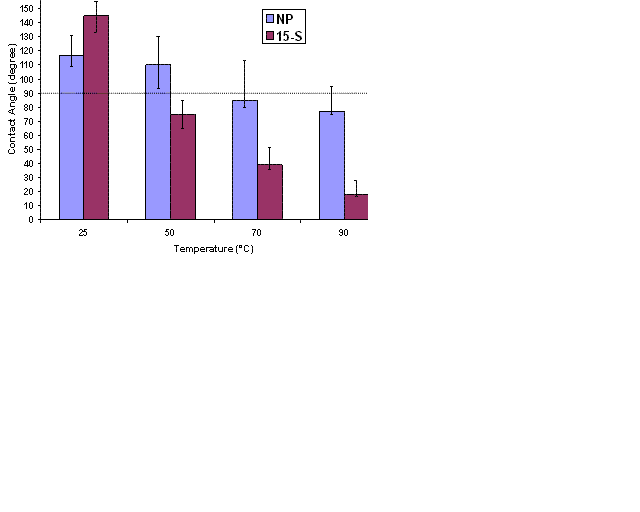
Figure 1: Effect of temperature on contact angle for non-ionic surfactants
The final contact angles for two surfactants (NP, 15-S) at their optimal salinities for four temperatures (25 oC, 50 oC, 70 oC and 90 oC) are reported in Fig. 1. The wettability varies along the surface of the plate because of the non-uniformity in initial oil contact with the plate. This leads to a range of contact angles observed after surfactant treatment. The error bars in Fig. 1 indicate the variation of contact angle between different drops in the same experiment. At a very low surfactant concentration (0.1 wt%), intermediate to water-wettability can be obtained for many of the surfactants studied. The contact angle was found to decrease with increase in temperature for all the surfactants. For these two surfactants the calcite plate remained oil-wet at 25oC but altered to water-wet at 70 oC and 90 0C. 15-S was found to alter the wettability the most among all the surfactants at 90 0C; the final contact angle was ~18o.
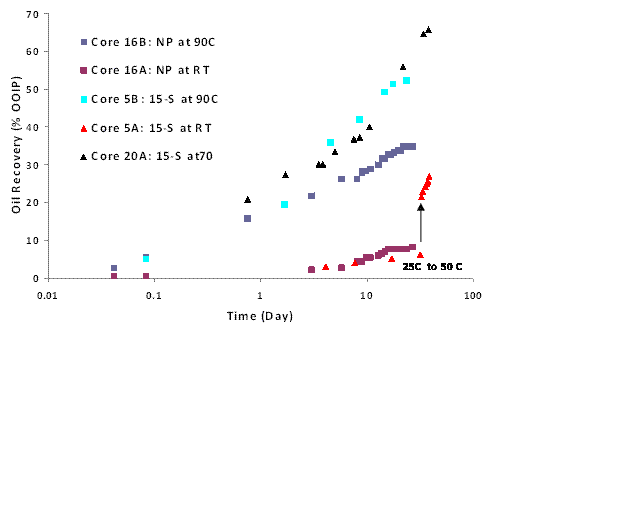
Figure 2: Oil recovery during imbibition with non-ionic surfactant solutions
Imbibition experiments were conducted by immersing initially oil-wet carbonate cores in different surfactant solutions. Fig. 2 shows the oil recovery in imbibition experiments for 10 md cores with two nonionic surfactants. The data plotted is an underestimate of the actual recovery because it does not account for the oil drops stuck to the core surface and the oil trapped in the macroemulsion (if any) below the separated oil layer. NP and 15-S recovered around 30% and 40% OOIP in about 10 days at 90 oC, respectively. Oil recovery exceeded 60% in about 30 days at 90 oC. At 25 oC, oil recovery is very small in 30 days, because the contact angles are high. The temperature of that imbibition cell was increased to 50 oC after 30 days. This increase in temperature also leads to increased imbibition of surfactant water and a bump in oil recovery. Oil recovery generally increased as the temperature increased because the rock became more water-wet and oil viscosity decreased. The same trend was seen with anionic surfactants.