Reports: GB3
44308-GB3 Synthesis and Photochemical Characterization of Hexacoordinate Silicon(diimine)3 Complexes
Background
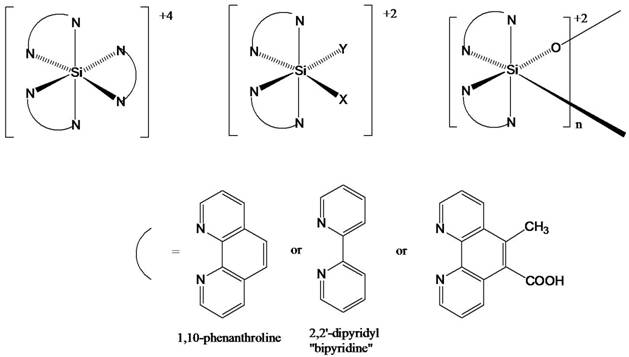
The main goals of our research have been to synthesize a library of hexacoordinate silicon complexes with multi-dentate polypyridyl ligands and to study their photophysical and photochemical properties (Figure 1). The ubiquitous Ru(diimine)32+ complexes (e.g. Ru(bipy)32+) have been the subject of many papers, due primarily to their extensively studied photochemical, photophysical, and electrochemical properties. The chemistry of the corresponding silicon analogs such as Si(bipy)34+ has been largely unexplored. The preparation of neutral tris-2,2'-bipyridinesilicon(0), Si(bipy)3, was first reported in 1963 by Herzog and Krebs.[i] This reactive compound reacts readily with iodine to form the stable Si(bipy)3I4 salt. [ii] The Si(bipy)3I4 salt can also be synthesized directly by melting SiI4 in the presence of 20 equivalents of the polypyridyl ligand for 18 hours. The lack of development in this field is perhaps due to the obvious lack of MLCT possibilities for the Si(bipy)3+4 salts, but we have found that the rich counter-ion to ligand CT possibilities combined with the extensive electrochemistry makes these compounds an important series to study.
Figure 1. Some representative hexacoordinate silicon complexes being studied
Improved Synthetic Procedure
We have developed a new synthetic procedure for species such as Si(bipyridine)3(PF6)4, Si(phenanthroline)3(PF6)4, and Si(terpyridine)2(PF6)4 which requires only stoichiometric amounts of ligand instead of the large excess amounts previously used. Furthermore, the reaction provides higher yields (~85%) in 1/10th of the reaction time. The large reduction in ligand waste will be very significant as we synthesize new species which possess more exotic/expensive polypyridyl ligands. More importantly, the new procedure also allows more flexibility for heteroleptic species such as Si(phendione)(phen)2+4 and Si(phendione)(phen)2+4.
Optical Studies
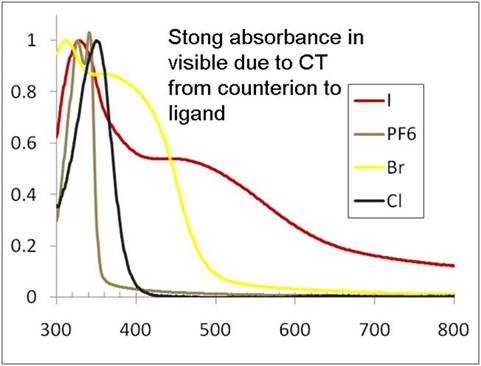
Despite the lack of MLCT possibilities, many of the Si(L)34+ halide salts are intensely colored especially in the solid state. UV-vis absorbance spectra of thin films of Si(bipy)3X4 (X = I, Br, Cl, PF6) (Figure 2) for example indicate the presence of a strong CT band from the counter-ion to a ligand based orbital, which is supported by DFT calculations.
Figure 2. Solid-state UV-vis absorbance spectra of various Si(bipy)3+4 salts.
Electrochemistry
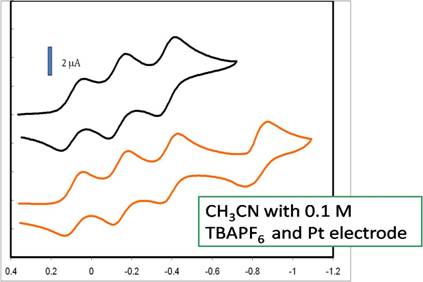
We continue to study the electrochemistry of the Si(bipy)34+ analogs using cyclic voltammetry. All of the species studied so far exhibit at least 3 reversible single electron reduction peaks. The fourth reduction peak to the neutral species usually indicates some irreversibility, such as shown for Si(bipy)3(PF6)4 in figure 3. It is believed that the reductions lead to electrons that are primarily ligand centered, and that the silicon center is still in a +4 oxidation state.
Figure 3. Cyclic voltammogram of Si(bipy)3(PF6)4 in CH3CN
Educational Impact
This project has enhanced the education of many undergraduates students, with 4 undergraduates receiving stipends from the ACS-PRF funds for summer research. In addition, the project has involved 4 high school students, who were paid stipends through various other programs. Three of the four have proceeded to pursue chemistry degrees in college, and the fourth is planning to once he graduates from high school. Once the electrochemistry of all the complexes is complete this fall, we will be submitting manuscripts which include many of the undergraduate and high school students as co-authors.
[i] Herzog, S.; Krebs, F., The preparation of tris-2,2'-bipyridine-silicon(0), SiBipy3. Naturwissenschaften 1963, 50, 330-1.
[ii] Kummer, D.; Gaisser, K. E.; Seifert, J.; Wagner, R., Contributions to the chemistry of halosilane adducts. XIV. Neutral ligand complexes of silicon with six N-donor atoms. Zeitschrift fuer Anorganische und Allgemeine Chemie 1979, 459, 145-56.