Reports: B1
47559-B1 Diels-Alder Reactions of Silyloxy Furans: Scope and Limitations
Progress:
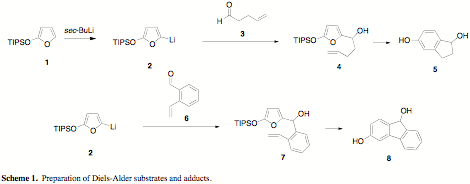
Initial results suggested that 5-lithio-2-triisopropylsilyloxyfuran (2) added to 4-pentenal (3) to produce a Diels-Alder substrate 4 that underwent cycloaddition at reasonable temperatures (Scheme 1). This has been difficult to replicate because of problems generating and handling 3. In our hands, 3 easily undergoes a self-aldol reaction during purification (distillation, silica gel chromatograhy). When we do obtain it pure, 3 rapidly trimerizes. While learning how to handle 3, we also investigated the synthesis and use of adlehyde 6. Addition of the lithiated furan 2 to 6, followed by gentle heating, produced a compound whose spectral features are consistent with fluorenol derivative 8. This example is particularly interesting as it demonstrates that we may be able to use this methodology to design tunable fluorescent probes that can be attached to biomolecules in ways that are complimentary to current methods. This will most likely become a primary focus for the next reporting period.
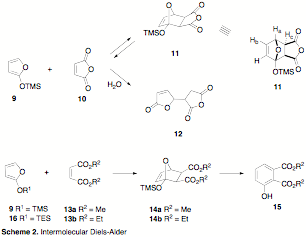
Another aspect of this project involved understanding how the size of the silyloxy group on the furan and the nature of the dienophile effect the rate of the Diels-Alder reaction. To this end, we have begun looking at the reaction of trimethylsilyloxyfuran 9 and various maleic acid derivatives (Scheme 2). Asaoka and co-workers reported that the reaction of 9 with maleic anhydride produced complex side reactions and very little (5%) of the product derived from 11 (i.e. like 15).[i] Triciclic 11 was never isolated or characterized. In our experiments, we can observe the rapid formation of a compound whose H1 NMR spectral characteristics are consistent with 11. If the reaction conditions are not rigourously dry, then over the course of a few hours we observe signals from a compound consistent with 12. It would appear that the Diels-Alder reaction has a low barrier to cycloreversion, and that the Michael adduct 12 is the thermodynamic product.
Interestingly, the H1 NMR signals corresponding to Ha (5.42 ppm) and Hc (3.38 ppm) in 11 appear as doublets, rather than the expected doublet of doublets (as Hb appears). Examining models (PC Model), the Ha-Hc dihedral angle for the endo-adduct should be approximately 45 û, whereas the same angle in the exo-adduct should be approximately 86 û. According to the Karplus correlation curve, the 86 û dihedral angle should produce a coupling constant close enough to zero that our instrument would not resolve it. It would appear, then, that the exo-adduct is the favored product. We are trying to find a more reliable method for assessing this stereochemistry.
Interestingly, dimethyl maleate (13a) reacts with 9 at rt somewhat slower (a few hours, as opposed to a few minutes) than 10, and diethyl maleate (13b) reacts slower still (days as opposed to hours). This trend of increasing ester size with slower qualitative kinetics is in line with our initial hypothesis that a steric interaction between the ester group and the silyloxy group may influence the rate of the Diels-Alder reaction. Oddly, the reaction of 9 with dimethyl fumarate has not produced any signs of cycloaddition. We are currently conducting quantitative kinetic experiments using H1 NMR in order to both quantitate the differences in rate between substrates and also extract thermodynamic parameters. To compliment these experiments, we have begun a computational study of these systems (B3LYP/6-311*) to help identify specific steric interactions.
We have also made 2-triethylsilyloxyfuran (16) and run some preliminary experiments to demonstrate that the kinetics are qualitatively slower than those of 9 with both maleic anhydride and dimethylmaleate. Reaction of 1 with maleic anhydride (10) is exceedingly slow, with only traces of the cycloadduct evident in the H1 NMR spectrum of the reaction mixture after several days. This result has led us to work on a protocol for using 2-triethylsilyloxyfuran (16) for our intramolecular work. Furan 16 can be made on reasonably large scale, it is reasonably stable, and appears to have usable reaction rates in the intermolecular reactions. The lithiation of 16 has not been reported. Unsurprisingly, lithiation with BuLi was unsuccessful, but some success has been achieved with LDA.
Impact:
This grant has opened up a new avenue of research at Gustavus Adolphus College, and has attracted much student interest. It has also provided the PI several examples with which to enrich his teaching. The fluorenol result has the potential to move this particular project well away from natural product applications and into areas that are fundamentally new to the PI. On an institutional level, this grant has been part of a successful effort to increase undergraduate research opportunities in the summers. This grant has had a particularly positive impact on the research environment at Gustavus. In combination with two institutional grants (Merck Institute for Science Education and Howard Hughes Medical Institute), the PRF grant has helped altered the research atmosphere at Gustavus profoundly.
Four different students have been trained under this grant, with two more anticipated for the next granting period. One student has already entered graduate school at the University of Minnesota, one is working for General Mills, and two (currently Juniors) have become very interested in research careers because of this experience. Three of the four students currently trained under this grant are women.
[i] Asaoka, M.; Miyake, K.; Takei, H. "Synthesis of 7-Hydroxyphthalides Starting from Unsaturated Lactones via 2-Tialkylsiloxufurans." Chem. Lett. 1977, 167-170.