Reports: AC9
46737-AC9 Multicomponent Droplet Growth in Supersonic Natural Gas Separators
Natural
gas supplies ~23% of As these devices are adopted, critical
questions remain regarding droplet growth in these complex vapor mixtures. The
goal of this work is to improve our fundamental understanding of water -
hydrocarbon droplet growth at cooling rates comparable of those found in the
novel separators. In particular, hydrocarbons can inhibit water condensation
because they wet the water surface while water cannot wet the hydrocarbon
surface. Since removing water is critical to preventing clathrate
formation, understanding co-condensation in this highly non-ideal system is
vital to the success of supersonic separator technology and efficient natural
gas recovery.
Our
experimental apparatuses include a series of supersonic nozzles with cooling
rates that match the supersonic separators. The characterization methods
include pressure trace measurements, infra-red spectroscopy measurements of the
gas phase, and in situ small angle
x-ray scattering measurements to directly follow the growing droplets. To date
we have made extensive measurements using the pure components and limited
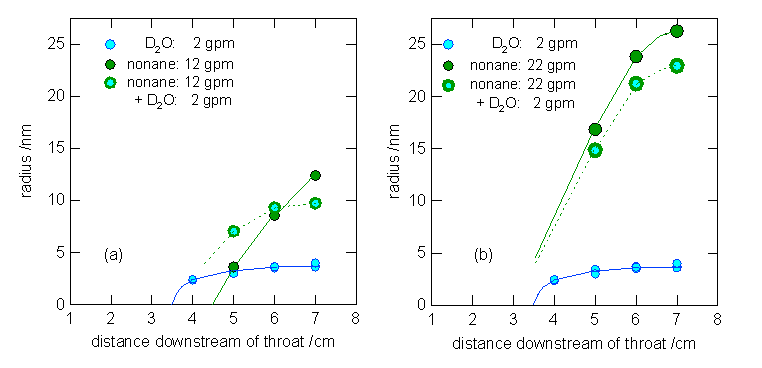
experiments with the binary system. Figure 1 illustrates our preliminary
position resolved droplet size measurements for pure nonane,
pure D2O and binary mixtures of these materials. In the experiments
presented here, the flowrate of D2O was
fixed at 2 g min-1 and the flow rate of nonane
was increased from 12 to 22 g min-1. In the first case, Fig. 1a,
pure nonane nucleation occurs further downstream than
D2O nucleation and the nonane droplets are
significantly larger than the D2O droplets. When the materials
co-condense, the droplets observed 5 cm downstream of the throat are
significantly larger than either the pure nonane or
pure D2O droplets. By the end of the nozzle, however, the pure nonane droplets are larger. Since binary nucleation is not
thought to occur, this behavior suggests that the nonane
condenses on the D2O droplets and that the final number density of
the aerosol formed during co-condensation is higher than that of the pure nonane droplets. For the higher nonane
flow rate, Fig. 1b, pure nonane nucleation occurs
upstream of pure D2O nucleation. In this case, the pure nonane droplets are always larger than the droplets formed
in the presence of D2O. This observation is consistent with the
formation of additional particles via D2O nucleation followed by
growth via nonane condensation. Additional
experiments, to better resolve the growth curves and to directly follow the
condensation of both species via infrared spectroscopy, are currently underway.
Figure 1: The growth of pure and mixed
droplets when nonane condenses (a) downstream of D2O and (b) upstream of D2O.