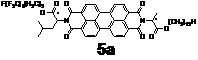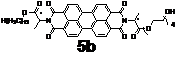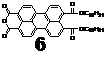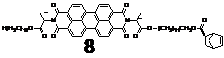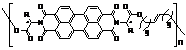Reports: G7
47744-G7 Structure Engineering of Side Chain Perylene Tetracarboxylic Diimide Diblock Copolymers
The progress report is given below in four parts.
(1) PDI phase tuning with symmetrically attached alkyl tails
We have successfully synthesized following compounds with symmetrically attached alkyl tails.
|
1. R1= CH3, R2=H 2. R1=CH3, R2=isopropyl 3. R1=isobutyl, R2=H |
4a. n = 8 4d. n = 14 4b. n = 10 4e. n =16 4c. n = 12 |
By using unsymmetrically placed steric-controlling groups (R1 and R2), we have achieved a new level of controlling hierarchical structure of PDIs. For instance, compound 1 features the tightest packed p-stacks (intra-stack separation 0.328 nm) among know PDI derivatives. More interesting, 3, 4c, 4d, 4e organize into a novel optically isotropic layered phase which undergoes a first order transition into the isotropic liquid state upon heating. Preliminary results suggest that in this phase PDI cores are microphase segregated from disordered flexible alkyl tails forming rigid layers. However, due to random and unsymmetrically placement of steric-controlling groups, the layers cannot maintain long-range molecular orientation order and can be considered ad random orientated at the length scale of visible light wavelength. Therefore the phase is optical isotropic. This phase can be considered as a new state of matter as it does not belong to crystalline, liquid crystalline, plastic crystalline or amorphous (liquid) phases.
(2) PDI phase tuning with unsymmetrically attached alkyl tails.
We have also prepared two PDIs with unsymmetrically attached alkyl tails as shown above. Due to the incompatibility between two different kind of flexible tails, it is expected that microphase segregation may take place between alkyl and (partially) perfluorinated alkyl chains (for 5a) and alkyl and tetraethylene glycol (for 5b). Indeed the microphase segregation occurs in both compounds when they self-assemble in the neat state. Small-angle X-ray diffraction results suggest that perfluorinated alkyl chains (or tetraethylene glycol chains) are microphase segregated from alkyl chains. This may offer the controllability of PDI structures at the nanometer level.
(3) Further development of unsymmetric synthesis of PDIs
We have developed a new synthesis pathway that can lead to the formation of unsymmetrically substituted PDIs. The key compound in this synthetical approach is an organic-soluble perylene monoanhydride (6). We designed this compound and prepared in a good yield via a facile procedure. Its applicability in unsymmetrically substituted PDIs was demonstrated.
(4) Structure tuning in PDI polymers.
As the part of effort of incorporating PDI units as the side groups of a polymer, we have synthesized the following PDI monomer. However, the free radical polymerization of 7 was not successful. No corresponding polymer was obtained.
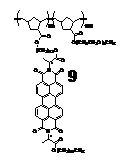
Thus we switched to ring-opening metathesis polymerization (ROMP) and prepared the corresponding monomer 8. ROMP of 8 was successful. Subsequently, a diblock copolymer 9 was also successfully prepared with a PDI of 1.18.
In addition, we have also successfully integrated PDI units into polymer main-chains by acyclic diene metathesis polymerization. The first two soluble nonracemic chiral main-chain perylene tetracarboxylic diimide polymers (shown below) were prepared and their solution aggregation behaviors were studied. It has been found that they self-assemble into helical structures even at highly diluted concentrations. These polymers could be used as the potential replacements for expensive fullerene derivatives as electron acceptors in organic solar cells.
R = methyl, isobutyl
We have made significant progress in structural tuning of PDI model compounds and have discovered a new type of structure of condensed matter. We also have successfully incorporated PDI units into polymers as both side groups and a part of main-chain. The next step is to study how the sub-phase of PDIs will affect/be influenced by block copolymer phase structure.