Reports: GB5
47286-GB5 Density Functional Theory and Car-Parrinello Molecular Dynamics Simulations of Ethanol Electro-oxidation Reaction on Bi- and Trimetallic Nanoparticles
Adsorption and decompositions of H2O and ethanol over PtnM clusters have been further systematically investigated by including external electric fields, and carrying CPMD simulations.
1. Adsorption and Dissociation of water over PtnM (n=6,9) clusters
To model sub-nano particles and discuss water behavior over alloy surface, PtnM (n=6 and 9; M=Pt, Sn and Ru) with given distances are chosen. Dissociation energy barrier (Ea) for the adsorbed water over Sn site of Pt6Sn is slightly lower than over Ru but remarkably lower than over the central Pt (Ea: 0.60 vs 0.67 and 1.14 eV). In spite of dramatically smaller than in the gas phase (5.26 eV), for all of three cases dissociation energies of adsorbed water on Pt6M (111) are still endothermic (0.50, 0.32 and 0.90 eV).
For the extended two-layer (111) surfaces Pt9M (M=Pt, Sn, Ru, Cu, Rh, Pd, and Re), the adsorbed water on the central M of the top layer exhibits atop configuration. Ea for the central Pt atom of Pt10 is slightly lower than that of Pt7 (1.07 vs 1.14eV), while the barriers for Sn and Ru are increased to different magnitude due to the second layer effect (0.78 vs 0.60eV for Sn; 0.96 vs 0.67eV for Ru). The energy barriers for Pt9M (M=Pt, Sn, and Ru) have the same trend as the above one-layer model. Once again the adsorbed water on Sn shows the lowest dissociation energy barrier, followed by Ru and Pt (Ea: 0.79 vs 0.96 and 1.07eV). It is interesting to note that the dissociation energy on Sn site is also the lowest (Ediss: 0.44 vs 0.61 and 0.67eV). This implies that from both kinetic and thermodynamic viewpoints Sn is the most active to water decomposition among the pure Pt and two PtM (M=Sn and Ru) alloys, which well supports the assumption of the bi-functional mechanism that Sn site accelerates the dissociation of H2O.
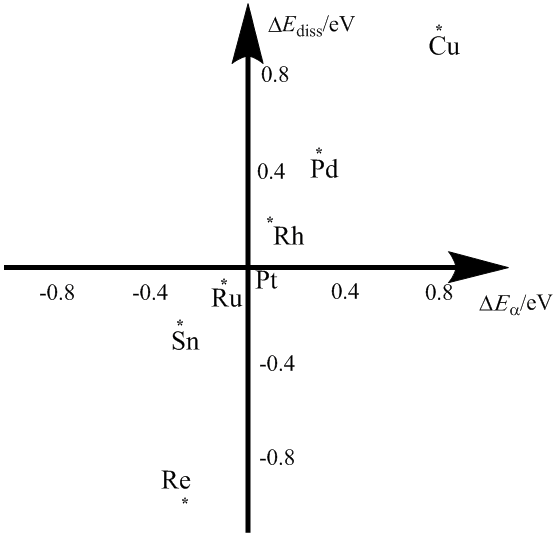
Figure 1. The plot of the relative dissociation energy against the relative energy barrier for water dissociation over the M site of Pt9M.
Water dissociation kinetic activity increases in the sequence of Cu < Pd < Rh < Pt < Ru< Sn <Re. In spite of different, the sequence is somewhat relevant to water adsorption strength order, Pt< Pd< Ru< Rh< Cu< Sn< Re. Taking Pt as a reference, the plot of relative Ea against relative Ediss for water dissociation over the investigated transition metals M (Pd, Ru, Rh, Cu, Sn, and Re) is illustrated in Figure 1. According to the plot, the bimetallic alloys PtM (M=Re, Sn and Ru) are more active to water dissociation both kinetically and thermodynamically, while the bimetals alloying with Cu, Pd and Rh are less active than Pt from both aspects.
2. External electric field effects Adsorption and Dissociation of water over PtnM (n=6,9) clusters
To discuss external electric field (EF) effects on water decomposition over PtnM(n=6 and 9; M=Pt, Sn, and Ru), external electric fields were applied to the adsorbed water in the z direction perpendicular to the top surface (xy plane) of the clusters PtnM. Electric field effect on water decomposition over Pt7 is opposite to those over Pt6Sn and Pt6Ru. Ea for adsorbed water over the former in the applied electric fields is considerably increased as compared to non-electric field (1.14eV), and such a variation does not change much with the strength (Ea=1. 40eV at EF=0.001au vs. 1.42eV at EF=0.01au) as well as with the direction (1. 40eV at EF=0.001au vs. 1.41eV at EF=-0.001au). However, Ea for water decomposition over the bimetallic clusters Pt6M (M=Sn and Ru) are in general decreased. For the decomposition over Sn site of Pt6Sn, upon the applied fields Ea is much lower by 23~52% than that without EF. Ea over Ru site of Pt6Ru is weakly affected by external electric fields, with a slight decrease in positive fields while little increase in negative field. It is interesting to notice that in the external electric fields PtSn still exhibits the most active kinetically to water decomposition among the three clusters. Electric fields were also extended to the two layer model Pt9M. For all of the three Pt9M (M=Pt, Sn and Ru) Ea was increased after applying electric fields. Under the given electric field PtSn again has the lowest energy barrier for adsorbed water to decompose.
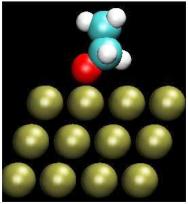
3. CPMD simulation of Adsorption and Dissociation of Ethanol over Pt (111)
Figure 2. Car Parrinello Molecular Dynamics simulation for ethanol adsorption and dissociation over Pt-based bimetallic nano-particles
CPMD simulation was carried out for ethanol and Pt slab with CPMD 3.9. The DFT method and the PPs used through the present simulation are the BLYP type of generalized-gradient approximation density functional, and the Troullier and Martins (TM) pseudopotentials, respectively. The system is represented by an orthorhombic supercell with dimensions of 9.58 x 8.30 x 15.81 Å, which contains a three-layer slab of Pt atoms with another six equivalent layers of vacuum representing. The vertical distance between oxygen atom and top layer ranges from 2.5 to 4.0 Å. The stable adsorption was only observed for the distance of 2.5 Å, yet no decomposition was displayed. The CPMD study will be extended by including electrolyte and bimetallic layers.
4. Impact on my career and on the students
The related research was continuously incorporated into teaching by involving two undergraduate students in the research, proving mini-projects to the students in Physical Chemistry and Computational Chemistry courses, which enable students to get aware of knowledge about renewable energies, QM software, and Linux supercomputers.