Reports: GB1
47142-GB1 Pd(0)-Catalyzed [3+2] Cycloadditions Involving Novel Epoxide and Aziridine Containing Trimethylenemethane Precursors
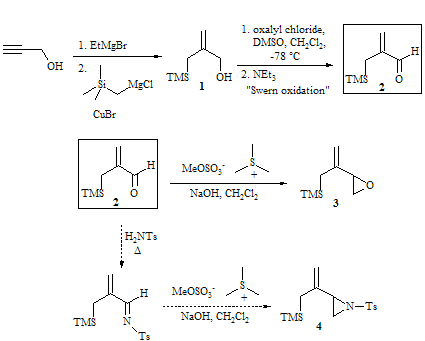
During the last year, two students and I have worked on this project. We have synthesized a large amount of compound 1, an important intermediate in the synthesis of our desired epoxide (3) and aziridine (4) precursors for the Pd(0)-catalyzed Trimethylenemethane (TMM) cycloaddition reactions. We have continued to increase the scale of the Swern oxidation and also for the reaction leading to compound 3. This summer we were able to make several grams of compound 3, purify it by Kugelrohr distillation, and proceeded to use this purified compound in the subsequent TMM cycloaddition reactions.
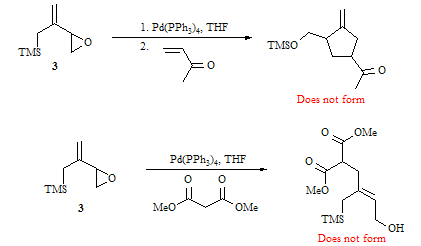
Previous efforts have shown that compound 3 does not undergo the Pd-catalyzed reactions shown below. Apparently, the epoxide does not open to form the intermediate π-allyl palladium complex, even though similar model systems by Trost have been shown to undergo epoxide opening. In an attempt to assist in the epoxide opening, we have added two different Lewis acids (TMSOAc and TMSOTf). It appears that these Lewis acids did promote the opening of the epoxide as there was no epoxide starting material returned after running the reaction. There is also evidence of the formation of a new product which has similar NMR chemical shifts to some products reported by Trost. However, we have been unable to identify the products that form. Further research is required to determine the identity of these new products.