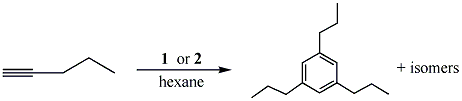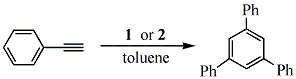Reports: GB3
47373-GB3 Catalytic Synthesis of Biologically Active Heterocyles with Organocobalt Complexes
The bulk of results described in this report were completed during a ten week period over June, July, and August of 2008. One undergraduate student, Jessica Pursel worked on this project and received a research stipend from this grant. Ms. Pursel did not continue to work on the project during the fall 2008 or spring 2009 semesters. Due to a scheduled renovation the principal investigators lab was closed beginning in May 2009, with an anticipated reopening date of July 31, 2010.
The principal investigator focused his time on preparing the starting materials, [(C6F5)C5H4]Co(CO)2 (1) and [(C6F5)C5H4]Co(COD) (2) for the catalytic reactions. Ms. Pursel continued the investigation of the catalytic activity. The initial investigation of the photolytic reaction between 1-pentyne and acetonitrile in the presence of 1 (eq 1) resulted in intractable
|
(1) |
results. Compounds 1 and 2 were shown to be catalytic via the reactions between 1-pentyne and the respective catalysts, in refluxing hexane, affording 1,3,5-tripropylbenzene and other isomers (eq 2). The products were indentified using GC/MS, however the yield was less than 10 %. The
|
(2) |
yield of the products were increased to approximately 60 % by refluxing in the higher boiling solvent, toluene. A second benzene derivative, 1,3,5-triphenylbenzene was prepared via the reaction of phenylacetylene and the respective catalysts, in refluxing toluene (eq 3). The product
|
(3) |
was isolated via recrystallization in a 64 % yield. The progress on this project was severely hindered by the fact that our NMR was damage and the subsequent repairs were put on hold until after the renovations are finished. It was decided that since the instrument was going to be stored anyway it would just be stored away early to save on cost.
When the principal investigators lab is reopened the investigation of the catalytic rates of the proceeding reactions will be continued and reactions to incorporate the nitrogen group into the products will be initiated.