Reports: GB1
47873-GB1 Rapid, Controlled Assembly of Polyenes for Studying Pericyclic Reaction Cascades
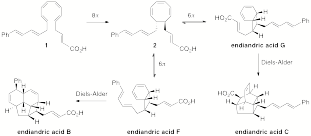
In collaboration with my physical chemist colleague at HMC Robert Cave, we have calculated ground state and transition state energies for the endiandric acid pericyclic reaction cascade shown in Scheme 1 using density functional theory methods. After comparing a wide range of computational methods on a truncated heptaene substrate (1 without the phenyl and carboxyl groups), we elected to use the B3LYP/6-31G(d) level of theory. Our preliminary calculations focused on estimating the relative energetics of endiandric acids B, C, F, and G and the transition states for F -> B and G -> C (Scheme 1). We have found that the activation energy for the endiandric acid F -> B Diels-Alder reaction is slightly lower than that for G -> C. Consideration of solvent does not significantly affect this trend, but replacing the carboxylic acid of 1 with a trifluoromethyl ester results in a lower transition state to form C. The corresponding methyl ester and methyl thioester are similar to the carboxylic acid. The series of calculations we have performed indicates that these systems are tractable to theoretical analysis and that we can make interesting predictions that have the potential to guide synthetic efforts. We are currently preparing a manuscript for the publication of these results.
Scheme 1. The pericyclic reaction cascade for endiandric acids B, C, F and G includes electrocyclizations and intramolecular Diels-Alder reactions.
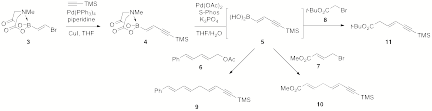
Our recent synthetic studies have focused on commercial, bifunctional alkene 3 as the starting point for all of our polyene cascade precursors. Bromoboronate 3 can be reliably converted to enyne 4 under Sonogashira conditions in a glovebox. This enyne can be deprotected in situ and immediately cross-coupled with a variety of electrophiles, including dienyl acetate 6, methyl 4-bromocrotonate (7), and tert-butyl bromoacetate (8). This has enabled us to rapidly assemble the advanced building blocks 9-11 (see Scheme 2).
Scheme 2. Preliminary results towards the biomimetic syntheses of endiandric acids A-G.
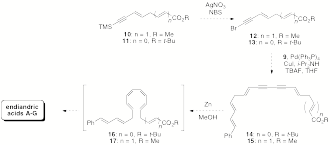
We are currently optimizing these Suzuki-Miyaura couplings and moving ahead to the remaining steps in the synthesis as shown in Scheme 3 below. We will continue to annually report our progress in poster presentations at spring ACS national conferences and hope to complete the synthesis and publish our results in the near future. My undergraduate students have done remarkable work on this project, especially Rachel Nishimura '09 who was so dedicated to this project that she continued her research during the summer following her graduation.
Scheme 3. Proposed biomimetic syntheses of endiandric acids A-G via diynes 14 and 15.
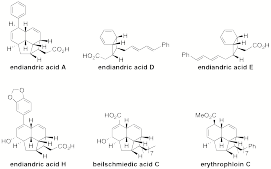
In summary, we have obtained enough computational data for a theoretical manuscript on the cascade and are close to completing the synthesis of endiandric acids A-G. We will then be able to move on to test our theoretical predictions and to synthesize biologically active members of this class of natural products such as endiandric acid H (anti-inflammatory), beilschmiedic acid C (antibiotic), and erythrophloin C (antitubercular). This work supported by the ACS-PRF grant has further enabled the submission of a NSF CAREER grant that was submitted in July 2009 and prominently features this project.
Figure 1.Structures of other endiandric acids and natural derivatives, including endiandric acid H, beilschmiedic acid C, and erythrophloin C.