Reports: G7
47926-G7 Graphene Nanoribbons: Synthesis and Self-Assembly of Nanostructured Materials
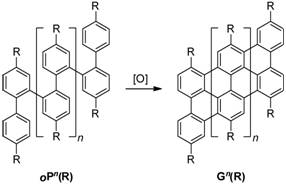
Over the past year, we have made significant progress in our project to develop oligomeric graphene nanoribbons as a new class of organic materials for applications in organic, supramolecular, and single molecule electronics. Briefly, the goal of this project is the development of synthetic methods toward ortho-phenylene oligomers (oPn), and their subsequent oxidative planarization to Gn. Graphene, a two-dimensional sheet of benzenoid carbon atoms, has recently attracted great attention due to its unusual and highly efficient charge-transport behavior. Compounds Gn represent the extreme limit of miniaturization of graphene-based structures, for use as fundamental models for comparison with theoretical approaches to the electronic structure of graphene, and as candidate molecular wires and self-assembled materials (e.g., liquid crystals).
Our principal effort in 2008-2009 has been to establish conditions for the synthesis of monodisperse ortho-phenylene oligomers based on an iterative series of Suzuki-Miyaura couplings. After determining that several published strategies for oligoarene synthesis are not suitable to this class of materials, we were able to develop a robust synthesis of oPn that has allowed us to synthesize a series of these oligomers with up to eight aromatic rings. With these molecules in hand, we have determined that they appear to exhibit interesting conformational behavior and we are very interested to determine how their electronic structure evolves with increasing length, particularly in the context of through-space delocalization. We are currently working to complete this study and anticipate publication in the coming months.
At the same time, we have carried out preliminary attempts to planarize both alkoxy- and alkyl-substituted oPn using conventional conditions for oxidative planarization (the Scholl reaction). To this point, our results have been mixed, consistent with a recent study published by King and co-workers (Tetrahedron 2008, 64, 11370). We have determined that direct planarization of oPn by treating with Lewis acid and oxidant does not appear to be a feasible route to Gn. However, we have managed to prepare a short Gn segment (G0) by a stepwise approach and are currently investigating whether this method can be extended to longer oligomers. We have also initiated an exploratory project to examine other catalyst options that avoid undesired rearrangements and decomposition.
Our PRF-G award has had a substantial impact on this project in particular and our research program in general. By its nature, synthetic organic chemistry is a cost-intensive research effort. Over the course of this project we have had to screen a variety of synthetic methods that require relatively expensive transition metal catalysts, ligands, and reagents. Given that we are a young group, we do not have an extensive stockpile of these materials from other projects and thus we have needed to purchase a large number of them. This grant has allowed us to aggressively pursue a variety of synthetic strategies, and we have built up a reasonable stock of these chemicals that benefits both this project and other efforts within the group. Further, the funding from the PRF has allowed us to purchase additional instrumentation for the group that are critically important to the project (UV/vis and fluorescence spectrometers). Having in-lab access to this instrumentation is, in my opinion, a significant educational benefit to the students, who get direct hands-on experience allowing them to explore the capabilities, limitations, and details of these techniques without pressure.
Over the course of the year, this grant has directly funded supplies for a post-doctoral fellow, a graduate student, several undergraduates, and myself, and has indirectly benefited my other group members (two graduate students and several other undergraduates) by freeing up our other funds. I anticipate that several publications will directly result from this year of funding, although we are still working to complete these studies. We have presented some of our results at statewide and national conferences, and anticipate many more such presentations in the coming year. We also obtained funding from the PRF to support an underrepresented minority student for the summer, which will be discussed in the separate report for the supplement.
Beyond the obvious scientific benefits, this grant has had a very measureable impact on my academic career. Preliminary results obtained directly as a result of this funding we used as part of a proposal submitted to the National Science Foundation, which was recently funded for four years as part of the American Reinvestment and Recovery Act. Obviously, this will be a sustaining grant for this project and we will therefore be able to support this effort over the medium-term.
While we have now committed most of the funds from this grant, we anticipate using the remaining balance to explore aspects of this project (e.g., new methods for oxidative planarization) that are not directly supported by our NSF funding. The PRF grant will therefore allow us to continue to develop new capabilities that we anticipate will be a foundation for our group for years to come.