Reports: B5
43890-B5 Gemini Surfactant Clay Intercalates for Triphase Catalysis
The ACS-PRF grant has given us the opportunity to conduct research in the area of triphase catalysis (TC), which is a unique type of phase transfer catalysis (PTC). Triphase catalysis generally facilitates the recovery of the catalyst via simple filtration or centrifugation and thus shows considerable potential for commercial application. Various types of solid supported catalysts, especially polymers, have been extensively studied as solid phase. However, the development of novel materials with higher mechanical and thermal stability than polymers for improved triphase catalysis is desirable.
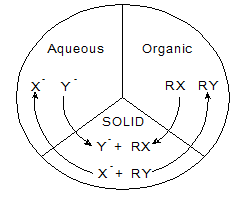
We have used hectorite clay from the smectite clay family (layered swelling clay) to support the catalysts through intercalation chemistry. These intercalates have been used as solid phase to carry out nucleophilic substitution reaction for converting n-butyl bromide to n-butyl chloride in a triphase catalytic system (see Fig 1 below).
n-butBr (org) + Cl - (aq) → Gemini-Clay Complex Calatyst → n-butCl (org) + Br - (aq)
Fig. 1 Schematic representation of a triphase catalytic reaction. The reactants from organic and aqueous phase come to the surface of the solid phase (hectorite clay-Gemini surfactant complex) and react.
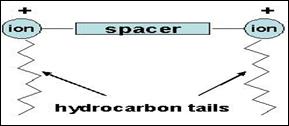
We have developed a series of novel clay supported catalysts using Gemini surfactants. Gemini surfactants comprise two N-alkyldimethylammonium bromide groups joined together by an alkyl spacer with the general formula (CnN(CH3)2Br)2X(CnCmCn), where n = number of carbons in the free N-alkyl chain and X is the bridging alkyl group containing m carbon atoms (see Fig 2 below).
Fig. 2 General representation of Gemini surfactant structure.
The significant difference from other classical mono-ionic surfactants is that the Gemini surfactants carry a double charge and provide the opportunity to test the effect of surfactant charge on the intercalation process and hence the catalytic activity of these intercalates.
In this work, we have synthesized and characterized a series of Gemini dicationic compounds C16-C2-C16, C16-C4-C16, C16-C6-C16, C12-C2-C12, C12-C4-C12, C12-C6-C12, C8-C2-C8, C8-C4-C8, C8-C6-C8 for application in triphase catalytic reactions using toluene as the organic phase and sodium chloride solution as the aqueous phase. We have used NMR analysis to characterize the Gemini surfactants. Also, all intercalated samples were studied by X-ray powder diffraction prior to kinetic studies.
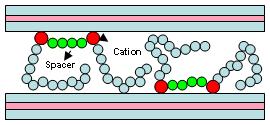
A simple schematic representation of Gemini intercalation is given below (Fig 3). However, due to the presence of two cationic entities on each Gemini molecule, the surfactant may have other choices of adsorption on the clay interlayer gallery surfaces and as a result, the basal spacing observed in some cases are larger than expected.
Fig. 3 The schematic representation of intercalated Gemini surfactants in the interlamellar space of hectorite clay.
By studying X-ray diffraction patterns and measured basal spacing we are seeing some differences which must be related to the length of the spacer or tail group of the Gemini surfactants intercalated in the interlamellar spacing of the clay. However, in terms of catalytic activity and rate of nucleophilic substitution reactions, two different trends were observed. In the C16-Cn-C16 Gemini series, we observed that the catalytic activity increases when the spacing group decreases in carbon number. An opposite effect was observed when C12-Cn-C12 or C8-Cn-C8 was used, which means that the catalytic activity increases when the spacing group became larger. In general, when the carbon number of the tail group is increasing, the catalytic activity is increasing. This may be related to the observed higher basal spacing, which facilitates substrate adsorption within the clay gallery surfaces and hence increases the reaction rate. However, an explanation for the catalytic activity of various spacing groups in C16 vs. smaller tail groups seems rather complex. One explanation is that the Gemini surfactants ions intercalated in the clay galleries may form non-ordered assemblies in which the alkyl chains and the head groups adopt random orientations with respect to the host layers. Based on our results, we tend to believe that the structure of the assemblies depends in part on the carbon number of the alkyl chains and the charge density of the hectorite clay layers.
The impact of this interdisciplinary area of research at SIUE has been significant. In particular, this research program has been a great source of experience for my undergraduate students. Currently, the PI is writing a manuscript for publication. The impact of this project on my research and my career has also been profound as I have been tenured and also promoted to Associate Professor partly because of my research activity that has stemmed from this grant.