Reports: G5
48160-G5 Chemical Vapor Deposition Design and Synthesis of Polymers for Alternative Energies
Fueled by the finite supply of petroleum, the search for alternative, more sustainable energies, such as the sun, is rapidly intensifying. Yet fundamental improvements in materials and methods are required to realize a competitive solar technology. In one type of solar cell known as the dye-sensitized solar cell (DSSC), a liquid electrolyte is typically integrated with a photosensitizer dye adsorbed on nanocrystalline titanium dioxide. There is a push to replace the liquid electrolyte with a solid electrolyte to minimize leakage and corrosive effects. However, DSSCs incorporating a solid-state polymer electrolyte yield low power conversion efficiencies as a result of poor filling of the mesoporous titania network attributed to poor wettability of polymer solutions in liquid-based processing. Thus, finding a means to completely fill the pores of the titania with a polymer electrolyte effectively is expected to provide a significant enhancement in the performance of polymer-based DSSCs. The objective of this starter project which is a first step towards this goal is to create polymer electrolyte systems based on hydrogels using a novel initiated chemical vapor deposition (iCVD) approach and integrate them into mesoporous titania. The rationale is that iCVD being a liquid-free process under a low pressure environment will overcome the issues of wet processing, and lead to tight contact between the polymer electrolyte and titania necessary for enhancing charge transport and efficiency in DSSCs.
iCVD is a method that enables
polymer films to be formed by relying on liquid phase polymerization
chemistries, only without the use of any liquids. An initiator and monomer
typically used in liquid based polymer synthesis are introduced as vapors into
an iCVD reactor maintained at low pressures of 10-1000 Pa; thermal activation
of the initiator using a series of heated filament wires at a temperature of
200-400 °C to generate primary free radicals; the radicals and monomer are
adsorbed onto a substrate kept cooled at a temperature of 0-30 °C to promote
surface adsorption; addition of monomer onto activated initiator sites to form
polymer chains, in tandem creating a uniform polymer film. iCVD is unique in
that both polymer synthesis and polymer film formation are combined into one
step. In addition, liquid-based challenges, including processing solubility
constraints, solvent-induced morphological changes, liquid surface tension and
lack of wettability are absent. Further, iCVD separates the activation and
substrate temperatures, thereby removing any temperature-induced material
stress, and allowing temperature-sensitive substrates to be coated without
damage.
To date, we have designed, assembled
and commissioned a state-of-the-art iCVD reactor system capable of synthesizing
polymer electrolytes for this project. It consists of a stainless steel reactor
chamber which can coat substrates of up to 200 x 200 mm on a deposition stage
that is temperature controlled using backside water flow; a vapor delivery
manifold to introduce multiple reactant feeds in the form of vapors; an
isolated support for suspending filament wires above the substrate stage; an
exhaust manifold to channel vapors exiting the reactor to a vacuum pump system;
a pump system that includes a dry mechanical pump with manual shut off valves
and foreline traps; a pressure controller connected to a reactor pressure gauge
and downstream throttle valve; and mass flow controllers and precision needle
valves to meter in reactant vapors.
With the iCVD reactor system, we
have successfully produced hydrogels of poly(2-hydroxyethyl methacrylate)
(PHEMA) and poly(ethylene glycol) (PEG) as potential polymer electrolytes for
DSSCs. FTIR and NMR spectroscopy have shown that iCVD PHEMA and PEG are
identical to polymers synthesized from liquid-based techniques, which is an
important distinction from other polymer CVD methods that do not typically
yield stoichiometric polymers. With PHEMA, we have begun to evaluate its
potential as a polymer electrolyte in DSSCs. Using an impedance analyzer, we
have obtained ionic conductivities in the range of 0.3-0.7 mS/cm with iCVD
PHEMA that incorporated propylene carbonate and γ-butyrolactone as
dielectric plasticizers and iodine/iodide as an ionic couple. In addition, we
have successfully used iCVD to deposit PHEMA and completely fill mesoporous
titania doctor bladed onto FTO glass by understanding iCVD deposition behavior
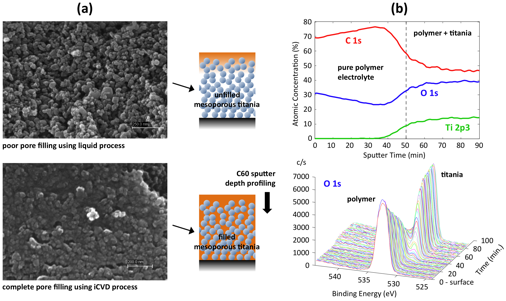
and coating formation. SEM and XPS using C60 sputter depth profiling clearly
demonstrates tight integration and contact between the polymer electrolyte and
titania, and confirms our hypothesis that iCVD processing will overcome
liquid-based challenges.
Integration of iCVD polymer electrolyte with mesoporous titania. (a) Cross-sectional SEM of mesoporous titania showing (top) lack of pore filling of polymer electrolyte using a liquid process and (bottom) effective pore filling of iCVD PHEMA. (b) XPS analysis with C60 sputter depth profiling of iCVD PHEMA filled titania showing along the thickness (top) elemental traces and (bottom) O1s high resolution spectra. Data clearly shows the transition from pure polymer at the surface to a mixture of polymer and titania within the structure that supports the pore filling seen with SEM.
Two Ph.D. graduate students are
currently working on this project. Our results have so far resulted in the
publication of a paper in Chemical Vapor Deposition on PHEMA hydrogels (Lau 2009a) and filing
of a patent application for the iCVD synthesis of PEG-based polymers (Lau
2009b) as well as contributing to a book chapter in The Encyclopedia of Chemical Processing
on "Vapor Deposition Polymerization" (Lau 2009c). In addition, two manuscripts
are in preparation, one on the mechanical analysis of iCVD PHEMA, and the other
on the integration of iCVD PHEMA and dye-sensitized titania as DSSCs. The work
has been presented by the students and PI at the 2008 AIChE National Meeting,
2008 ACS Fall Meeting and 2008 International Conference on Hot Wire Chemical
Vapor Deposition. Importantly, this research project has successfully led to
the PI being awarded the NSF CAREER Award in December 2008, which expands on
this project towards engineering polymer electronic materials for alternative
energies, and aligns well with the spirit of the ACS PRF starter grant to
stimulate further research and support. This project will continue in the
future with integrating iCVD PEG into mesoporous titania as well as evaluating
DSSC performance on solar cells integrating the iCVD polymer electrolytes.