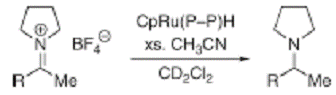Reports: B3
42806-B3 Compounds of Bidentate Phosphines with Metallocene Backbones: Electrochemistry and Catalysis
Undergraduates in my lab have continued to investigate the properties of bidentate phosphines that have metallocene backbones. To date our work has focused on the synthesis, electrochemistry and structural analysis of compounds containing either
1,1'-bis(diphenylphosphino)metallocene (metallocene = ferrocene (dppf), ruthenocene (dppr) or osmocene (dppo)) or 1,1'-bis(dialkylphosphino)ferrocene (alkyl = i-propyl (dippf), cyclohexyl (dcpf) or t-butyl (dtbpf)).
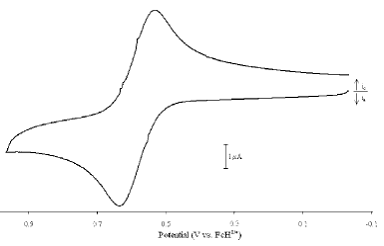
In the past year, we have completed an investigation of the asymmetric compound 1-diphenylphsophino-1'-ditertbutylphosphinoferrocene (dppdtbpf).1 We examined the electrochemistry of metal complexes containing the dppdtbpf ligand (Figure 1). We also examined a series of phosphine sulfides and phosphine selenides based on dppdtbpf.
Figure 1. Cyclic voltammogram of [Au2Cl2(dppdtbpf)].
We also completed a project in collaboration with Professor Jack Norton of Columbia Univeristy.2 We examined the effect of changing the bidentate phosphine on the hydride transfer ability of ruthenium hydrides. We determined that CpRu(P-P)H (P-P = dppf, dppr or dppo) compounds were very poor hydride transfer agents toward iminium cations (Figure 2). The alkyl derivatives, CpRu(P-P)H (P-P = dcpf or dippf), did not transfer a hydride to iminium cations. The compounds were to basic and deprotonated the iminium cation to give CpRu(P-P)H2.
Figure 2. Hydride transfer ractions of CpRu(P-P)H.
1) Kahn, S.L.; Breheney, M.K.; Martinak, S.L.; Fosbenner, S.M.; Seibert, A.R.; Kassel, W.S.; Dougherty, W.G.; Nataro, C. Organometallics 2009, 28, 2119.
2) Shaw, A.P.; Norton, J.R.; Buccella, D.; Sites, L.A.; Kleinbach, S.S.; Jarem, D.A.; Bocage, K.M.; Nataro, C. Organometallics 2009, 28, 3804.