Reports: AC1
47575-AC1 A Greener Phase-Switch Concept for Multistep Synthesis Using Boronic Acids as Productive Tags
There are increasing environmental pressures to
improve the sustainability of reaction processes in synthetic organic
chemistry. In response to these
demands, new techniques and strategies are needed in order to accelerate and
facilitate the synthesis and purification of organic compounds while minimizing
the waste of solvents and chromatographic supports. Indeed, much of the wastes produced in organic reactions
originate from the extensive use of silica gel and solvents employed in the
chromatographic purification.
Phase-switching strategies are very attractive as a means to avoid
chromatographic purification. In
phase-switch chemistry, reactions take place conveniently under homogeneous
conditions, and product separation is facilitated by a liquid partition or a
precipitation–filtration operation.
All known phase-switching strategies require two chemically unproductive
steps: attachment of the tag to the substrate, and detagging of the product at
the end. The latter operation
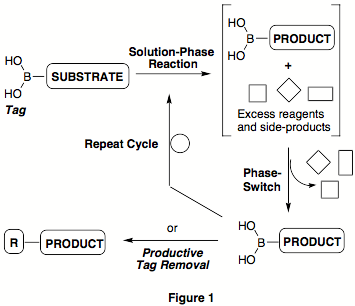
often leaves an undesired remnant (or "trace") of the phase-switch tag. We designed a new phase-switching
strategy involving the boronic acid functionality as a productively convertible
tag (Figure 1). Rather than
cleaving the extraneous tag at the end of a synthetic sequence, as in other
phase-switch systems, the boronic acid can be derivatized productively using
the wide range of selective transformations known for this class of compounds. Because of the commercial
availability of hundreds of functionalized boronic acids that can serve as
potential substrates in many synthetic applications, this phase-switch system
can also circumvent the tag attachment step.
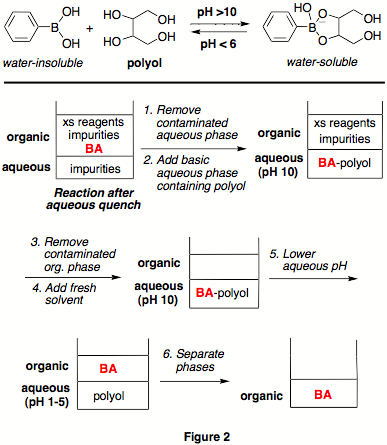
We
developed a liquid-liquid, water-organic phase-switching system by exploiting
the known ability of boronic acids to form strong complexes with polyol
additives at high pH (Figure 2).
We sought a polyol additive that would be polar enough to efficiently
"phase-switch" hydrophobic boronic acids to an aqueous phase and be completely
insoluble in organic solvents so as to avoid contamination of the organic layer. Toward this end, the ability of
different polyol additives to phase-switch the hydrophobic biphenylboronic acid
from ethyl acetate to basic water was rapidly evaluated, and the hexol
sorbitol, a cheap and non-toxic commodity chemical, was found to be the most
efficient at a pH of 10 and over.
Further optimization identified sodium carbonate as a preferred, milder
base. The efficiency of this
system was demonstrated in a control experiment whereby a mixture including p A
phase-switching system employing boronic acids as phase tags must be
complemented with a broad repertoire of chemical transformations tolerant of
this functionality. In this
regard, we have planned a number of simple transformations of model unprotected
boronic acids with a phase-switch purification. Remarkably, we found that
alcohol oxidations, carbonyl reductions, carbodiimide-promoted esterification,
carbonyl addition reactions with organometallic reagents, and even a sequence
of aldehyde alkynylation and alkyne-azide cycloaddition can all be realized in
good to high yields and high purify after the phase-switch purification.
In
the planning of a phase-switch synthetic cycle to a desired compound class, the
initial substrate is selected from a broad choice of commercial boronic acids,
and that functionality is preserved until the last step of productive tag
removal. To illustrate the virtues of the boronic acid functionality as a
productive tag, we optimized a diversity-oriented chromatography-free synthesis
of polysubstituted biaryl-containing isoxazolines involving a variety of common
reactions such as Wittig olefination, [3+2] nitrile oxide cycloaddition, and
amide couplings. The final productive clevage of the boronic acid tag was
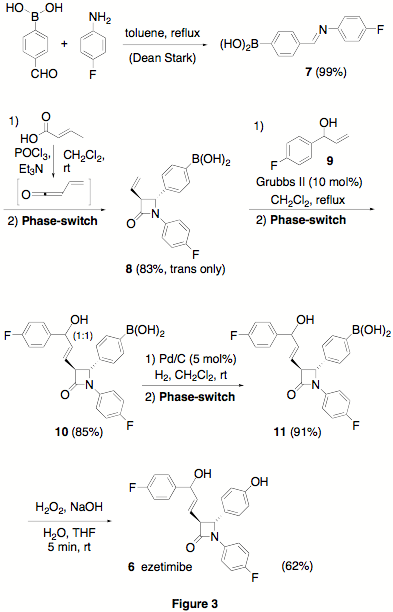
effected by a Suzuki cross-coupling reaction. Likewise, to demonstrate the
value of this productive phase-switch concept in target-oriented synthesis, we
put it to test toward a racemic synthesis of ezetimibe, (Zetia), a commercial