Reports: B7
44893-B7 Aggregation and Liquid Crystal Properties of Two Chromonic Systems
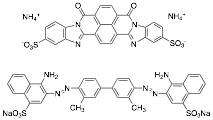
The goal of the grant was to investigate two new dyes that form chromonic liquid crystals, Bordeaux Dye and Benzopurpurin 4B (top and bottom, respectively, in the figure below). The former has a structure quite different from the other dyes previously studied and the latter is reported to have a liquid crystal phase at very low concentrations. These studies were essentially completed a year ago, but one final investigation was performed with Bordeaux Dye.
Bordeaux Dye
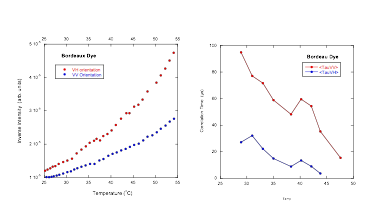
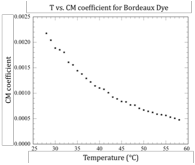
Light scattering measurements were performed to investigate its aggregation behavior. The results demonstrate that the aggregation process does not start at a critical temperature, unlike recent reports from light scattering experiments on the chromonic liquid crystal Cromolyn, where a critical aggregation temperature was detected about 10°C above the transition. Both the inverse scattering intensity and the correlation times (for both vertical-vertical and vertical-horizontal polarization geometries) show no onset in aggregation behavior within 20 °C of the start of the coexistence region (see figures below).
Magnetic Birefringence Measurements
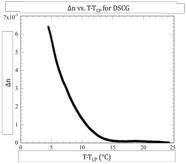
A new line of experimentation was initiated this year that involved measuring the birefringence induced by a magnetic field in the isotropic phase. As the aggregates grow in size, the magnetic field induces more and more order, increasing the birefringence of the material. Such measurements are an excellent way to track the aggregation process. Since these materials are not magnetic at all, extremely strong magnetic fields are required. In collaboration with a group at Kent State University, we applied for and were granted magnet time at the National High Magnetic Field Laboratory in Tallahassee, FL. Three separate trips were made to the laboratory, and several chromonic liquid crystals were investigated. We are still in the process of analyzing the results, but a few conclusions are quite clear. First, Bordeaux Dye, Sunset Yellow FCF, and Blue 27 all fail to show a critical aggregation temperature in the isotropic phase (see left figure below). Only Cromolyn displayed such a critical temperature about 10°C above the transition (see right figure below). Thus the recent report of a critical aggregation temperature in Cromolyn as revealed in light scattering experiments has been corroborated through our absorption coefficient measurements (reported a year ago) and now our magnetic birefringence measurements. More important, our light scattering results on Bordeaux Dye and our magnetic birefringence measurements on three different systems including Bordeaux Dye reveal that a critical temperature is not present in all of these systems. For some reason, Cromolyn is unique!
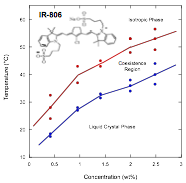
An Infrared Absorbing Chromonic Liquid Crystal
A recent publication made reference to the fact that an infrared absorbing dye, IR-806, forms a chromonic liquid crystal phase. Since this material represents a different structure (see figure below) and a different absorption band from all the other dyes previously investigated, we undertook to characterize its aggregation and phase behavior. The phase diagram for IR-806 is similar to that of Benzopurpurin 4B in that the liquid crystal phase appears at very low concentrations (see figure below).
In addition, all of the slow dynamics observed with Benzopurpurin 4B are also true for IR-806. The working hypothesis therefore is that the aggregates in this system are quite large compared to the other systems. Two other properties of this system are different from other chromonic liquid crystals. First, IR-806 photo-bleaches slowly, so experiments have to be done quickly with the samples kept in the dark as much as possible. Second, aggregation produces absorption peaks that are distinct from the molecular absorption peak. The figure to the left below shows a low concentration where monomers dominate . The figure to the right below shows a high concentration where aggregates dominate. We are in the process of analyzing the changes in the absorption spectrum in this system in hopes that it will reveal significantly more information than in other systems where the monomer and aggregate peaks cannot be resolved from one another.