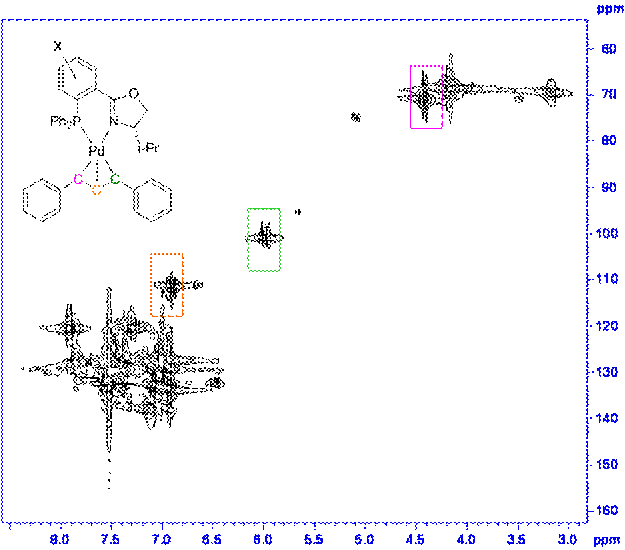Reports: B4
46722-B4 Hammett Studies of P,N-Chiral Ligands
Summary
We have successfully synthesized a number of our planned 5'-PHOX ligands using the Buckwald-Stoltz coupling procedure. These new ligands have been characterized and then converted into the corresponding 1,3-diphenylallylpalladium complexes for NMR study. Using 2D COSY and HMQC techniques we were able to assign all the necessary H1, H3, C1, C3, and P signals for both the exo and endo complexes. The chemical shift data was taken from the corresponding 1D spectra. Hammett analysis using various substituent constants was carried out. The analysis and interpretation is continuing. We are continuing to synthesize a few more ligands (specifically the acetyl substituent). We plan to study the asymmetric reactions with these ligands next.
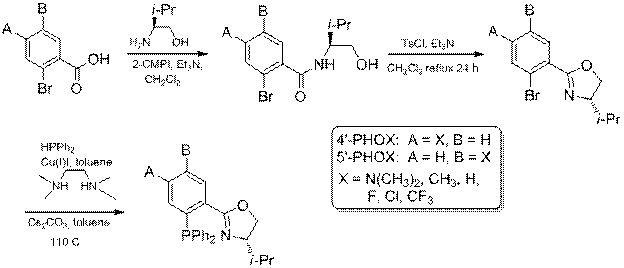
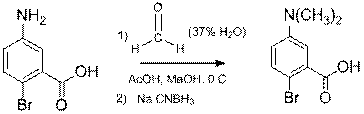
The 5'-PHOX ligand synthesis proceeded as shown in Scheme 1. For the 5'N(CH3)2 substituent was prepared from the available 5-amino-2-bromobenzoic acid (eq 1). These reactions proceeded in good to high yield and all intermediates were fully characterized. We also resynthesized the 4'-PHOX ligands we had made with our previous route, along with the new 4'-CF3 ligand by the route in Scheme 1.
Scheme 1. General Synthesis using Buchwald-Stoltz Coupling
|
Eq. 1 |
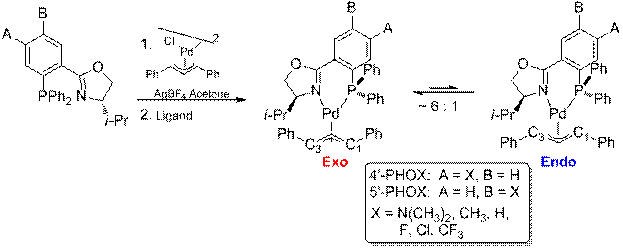
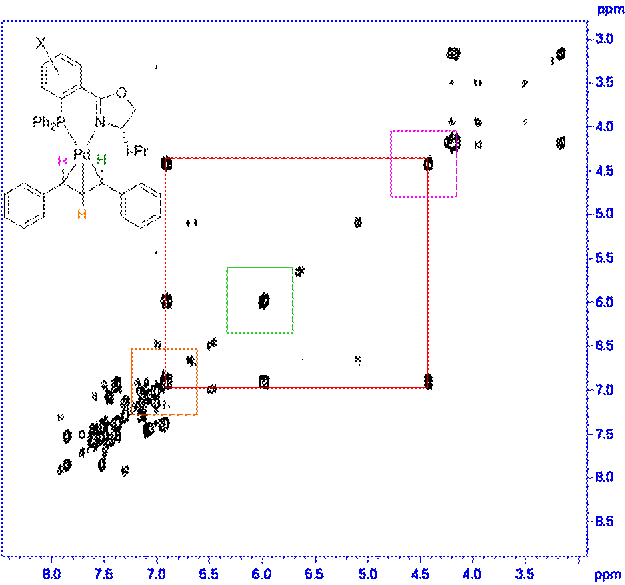
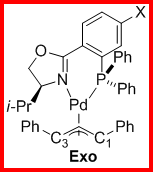
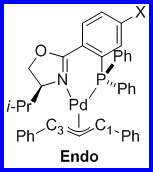
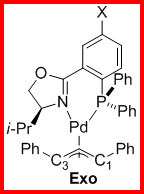
The ligand 1,3-diphenylallylpalladium complexes for both the 4' and 5' ligands were prepared as shown in Scheme 2. For all the complexes we examined the ratio of exo to endo intermediates was about 6:1 (by 1H and 31P NMR integration) and did not vary in any trended way with the identity of the substituents. The 1H and 13C assignments for each complex were made using the COSY (Figure 1) and HMQC (Figure 2) spectra. The 13C assignments were taken from the 1D spectra and are tabulated in Table 1 (4'-PHOX complexes) and Table 2 (5'PHOX complexes. The data for 4'PHOX ligand complexes were redone to best facilitate a direct comparison between the isomeric substituents and eliminate as many possible sources of variation as possible. The spectra for the 4' and 5' isomers were typically taken on consecutive days, for example, to minimize any changes in the spectrometer conditions.
Scheme 2. Ligand 1,3-Diphenylallylpalladium Complex Synthesis
Figure 1. Sample COSY of complex.
Figure 2 Sample HMQC of complex.
Table 1. 4'-PHOX 1NMR Data
Table 2. 5'-PHOX NMR Data
|
|
|
||||||||||
| Chemical Shift (ppm) | Chemical Shift (ppm) | ||||||||||
| C1 | C3 | P | C1 | C3 | P | ||||||
| 70.98 | 101 | 21.10 | 75.02 | 95.6 | 25.30 | ||||||
| 69.98 | 100 | 19.19 | 73.78 | 94.9 | 23.76 | ||||||
| 70.70 | 101 | 19.71 | 74.61 | 95.4 | 24.20 | ||||||
| 70.82 | 101 | 20.38 | 74.80 | 95.5 | 24.71 | ||||||
| 71.41 | 102 | 20.20 | 75.41 | 96.1 | 24.65 | ||||||
| 71.92 | 102 | 21.15 | 76.11 | 96.4 | 25.49 | ||||||
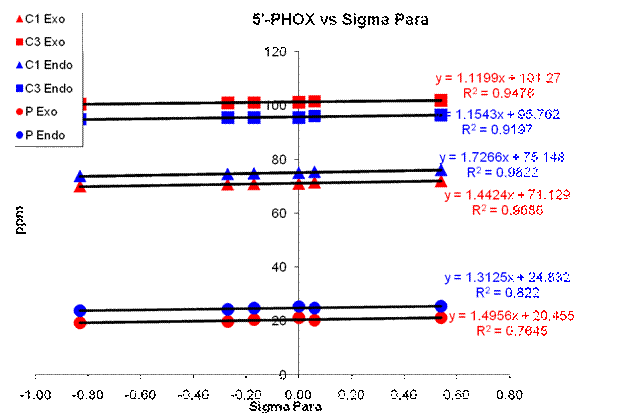
A sample Hammett plot is shown in Figure 3. We are still working on the interpretations and conclusions to draw from our Hammett analysis. In addition we have just recently completed repeating the NMR work on the Cl ligand (4' and 5') to add to this analysis. We plan to continue with this work and begin the asymmetric reaction studies with these ligands to correlate the NMR data to ee outcomes.
Figure 3. Sample Hammett Analysis Plot.