Reports: AC1
44949-AC1 Development of Photochemically Removable Groups
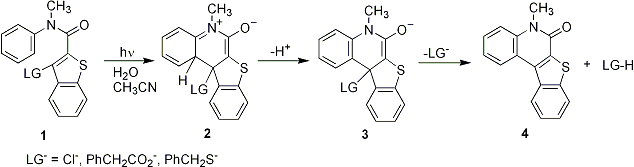
We previously reported on the generation of zwitterionic intermediates via photochemical electrocyclic ring closure reactions of acrylanilides bearing allylic leaving groups. Deprotonation of the zwitterionic intermediates produced enolates which then expelled various leaving group anions such as carboxylates and phenolates. Quantum yields ranged from 0.05 0.09 and the inefficiency in the reaction was largely due to radiationless decay of the singlet excited state, which effectively competed with electrocyclization. The triplet excited state yields were 0.15-0.20, but the triplet was unreactive, probably due to free rotor decay. Since then we examined the photochemistry of aromatic anilides which incorporate benzothiophene, e.g. 1. Direct photolyses result in expulsion of leaving groups such as chloride, carboxylate, and thiolate in quantitative yields and produce 4 as coproduct. Quantum yields are 0.22 at 310 nm for expulsion of chloride. In aerated solvent the quantum yield is 0.078, which suggests that the triplet excited state is the reactive excited state. Work is underway on incorporating glutathione as leaving group to regulate the redox potential of cells in biological studies. The wavelength will be shifted to 350 nm by introducing a benzoyl group in the anilide moiety, and intersystem crossing will be promoted by incorporating heavy atoms into the benzothiophene ring.