Reports: AC7
45891-AC7 Controlled Polymerization of Renewable Cyclic Esters: Catalyst Design and Polymerization Mechanisms
Decreased reliance on petroleum feedstocks for plastics production will be an important contributor to sound long-term national and international energy policy. As such, the petroleum research field will ultimately need to adapt to this inevitable shift from current practices. An attractive strategy is to develop new methods for the synthesis of polymers with useful properties from renewable starting materials. Towards this end, new and innovative methods are sought for the conversion of molecules provided by plants into compounds that can be catalytically transformed into sustainable plastics. We have taken an interdisciplinary approach that integrates chemical synthesis and structural definition of new catalysts, monomers, and polymers, with particular emphasis on mechanistic studies of polymerization catalysis via synergistic use of experimental and theoretical methods. Specific aims are to (i) develop a new class of polymerization catalysts based on a recently proposed activated monomer mechanism, (ii) uncover important and fundamental mechanistic information concerning these catalysts, and (iii) develop syntheses of new polymeric structures with useful properties from new monomers derived from agricultural products.
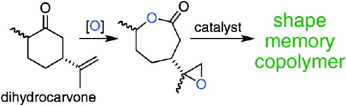
In work supported by the ACS-PRF grant completed in 2009, the natural product dihydrocarvone, found in caraway oil, was oxidized to an epoxylactone on a multi-gram scale. The resulting epoxylactone was used as a multi-functional monomer and crosslinker in ring opening polymerizations (Figure). While homopolymerization using diethylzinc and tin(II) 2-ethylhexanoate gave only low molecular weight oligomers (apparent Mn less than 2.5 kg/mol), copolymerizations of ε-caprolactone and 0.3 to 50% of the epoxylactone gave flexible crosslinked materials on a multi-gram scale in a one-step synthesis. The gel fraction of these copolymers was determined. These copolymers showed near perfect shape memory properties even after repeated bending.
In separate work, the hydrolytic degradation of polylactide–polymenthide–polylactide triblock copolymers (37 °C, pH 7.4) was compared to that of the component homopolymers. In addition to mass loss and water uptake measurements, size exclusion chromatography (SEC), 1H NMR spectroscopy, differential scanning calorimetry (DSC) and mechanical testing were used to monitor property changes during degradation. The rate of copolymer degradation was significantly influenced by the molecular weight of the polylactide end blocks. Mass loss of the polylactide homopolymer and the copolymer samples was observed once a decrease in the total molecular weight of the samples of 20% occurred. 1H NMR spectroscopy and DSC analysis of the copolymers during degradation revealed that the released oligomers contained mostly polylactide. After initial water uptake in which the mechanical properties were compromised to an extent, the Young's modulus and elongation at break of the biorenewable copolymers remained relatively unperturbed for up to 16 weeks, with significant retention of thermoplastic elastomeric properties for up to 21 weeks.
Continuing studies of triblock copolymers derived from renewable resources, a series of polylactide–polymenthide–polylactide triblock copolymers containing either amorphous poly(D,L-lactide) or semi-crystalline, enantiopure poly(L-lactide) or poly(D-lactide) end segments were synthesized. Small-angle X-ray scattering and differential scanning calorimetry data were consistent with microphase separation of these materials. The Young's moduli and ultimate tensile strengths of the semi-crystalline triblock copolymers were two and three-fold greater, respectively, than their amorphous analogs. Symmetric (50:50) and asymmetric (95:5) blends of the triblock copolymers containing two different enantomeric forms of the polylactide segments formed stereocomplex crystallites, as revealed by wide-angle X-ray scattering and differential scanning calorimetry. Compared to the enantiopure analogs, these blends exhibited similar ultimate elongations and tensile strengths, but significantly increased Young's moduli. Collectively, these results demonstrate that the properties of these new biorenewable thermoplastic elastomers can be systematically modulated by changing the stereochemistry of the polylactide end blocks.
Finally, in work aimed at developing new selective cyclic ester polymerization catalysts, indium trichloride, benzyl alcohol, and triethylamine (without an added 'directing' multidentate ligand) were found to generate a catalyst in situ for the room temperature polymerization of D,L-lactide affording highly heterotactic polylactide of controlled molecular weight and narrow molecular weight distribution.
Figure. Use of the renewable resource dihydrocarvone for the synthesis of shape memory copolymers with epsilon-caprolactone.