Reports: G10
47332-G10 Synthesis, Structural Characterization and Property Optimization of Novel Antimonides and Bismuthides Promising Materials for Thermal-to-Electric Energy Conversion
The compounds of the least electronegative metals from groups 1 and 2 with the more electronegative metals and metalloids, commonly known as Zintl phases, have attracted much interest over the years. In particular, very recently, detailed studies on such ternary antimonides with complex structures, such as Yb14MnSb11 and CaxYb1–xZn2Sb2 have shown that Zintl phases exhibit high thermoelectric figures of merit (ZT) and therefore, can be used as materials for direct thermal-to-electric energy conversion. As the thermoelectric properties are closely related to the crystal structures, the search for new pnictides with complicated structures, has gained renewed interest.
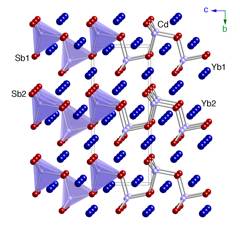
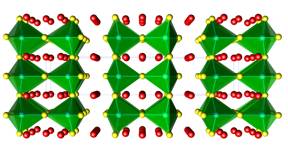
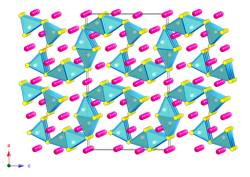
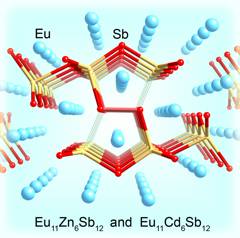
The research in our group has been inspired by these recent discoveries, which motivated us to conduct systematic studies of the fundamental trends in electronic and crystal structures of alkaline- and rare-earth metals antimonides and bismutides. A number of new Zintl phases have already been synthesized and structurally characterized, including Yb2CdSb2 and Ca2CdSb2 (Figure 1), Eu10Cd6Bi12 (Figure 2) and Ba11Cd8Bi14 (Figure 3), Eu11Cd6Sb12 and Eu11Zn6Sb12 (Figure 4). As a part of these ongoing systematic investigations in the A–M–Pn systems, where A = divalent alkaline- or rare-earth metals, M = Mn, Zn, Cd, Pn = pnicogen, we embarked on studying the Ba–Cd–Sb phase diagram. For a long time, there have been known only two compounds BaCd2Sb2, and BaCdSb2, but we recently discovered the new complex Zintl phase Ba11Cd6Sb12. Herein, we describe the continuation of our studies, and report on the synthesis, structural characterization and electronic band structure calculations of the new Zintl phase Ba3Cd2Sb4. It crystallizes with a complex monoclinic C-centered structure, which is composed of Cd centered Sb tetrahedra that share edges. Attempts to synthesize analogs of the title compound resulted in four mixed-cation Ba3–xAxCd2Sb4 phases, where A = Ca, Sr, Eu, Yb.
Crystals of the new ternary compound Ba3Cd2Sb4 were obtained from a reaction of Ba, Cd and Sb metals in Pb flux. Analogous reactions were also undertaken in the systems A–M–Pn, where A = Ca, Sr, Eu, Yb; M = Zn, Cd; and Pn = Sb, Bi, but did not yield any compounds, isostructural with Ba3Cd2Sb4. Successful were only reactions with mixed A–Ba cations, which produced Ba3–xAxCd2Sb4 solid solutions with narrow homogeneity ranges.
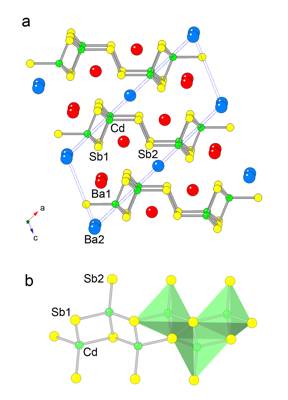
The crystal structure of
Ba3Cd2Sb4 was
established by X-ray diffraction. It is best described as a polyanionic
[Cd2Sb4]6– sub-lattice made up of CdSb4
tetrahedra and Ba2+ cations enclosed
between them, as shown in Figure 5. The CdSb4 tetrahedra share
corners to form ![]() [CdSb3]
chains, running along the crystallographic b-direction. Similar structural fragments are found in many Zintl
phases as well, for example
[CdSb3]
chains, running along the crystallographic b-direction. Similar structural fragments are found in many Zintl
phases as well, for example ![]() [AlAs3]6–
in Ca3AlAs3, and
[AlAs3]6–
in Ca3AlAs3, and![]() [SnP3]5–
in Sr5Sn2P6, to name a few. In Ba3Cd2Sb4,
the
[SnP3]5–
in Sr5Sn2P6, to name a few. In Ba3Cd2Sb4,
the ![]() [CdSb3]
chains are "dimerized" through shared vertices and edges, resulting in [Cd2Sb4]
double chains (Figure 5b). The latter are further connected to the neighboring
chains via exo-Sb–Sb bond
to form two-dimensional layers. The
[CdSb3]
chains are "dimerized" through shared vertices and edges, resulting in [Cd2Sb4]
double chains (Figure 5b). The latter are further connected to the neighboring
chains via exo-Sb–Sb bond
to form two-dimensional layers. The ![]() [Cd2Sb4]6–
layers propagate parallel to the [20
[Cd2Sb4]6–
layers propagate parallel to the [20![]() ]
plane and are separated by the large Ba2+
cations (shortest interlayer distance greater than 4.7 ). The Cd–Sb
distances fall in the narrow range from 2.9125(4) to 2.9531(5) and the
Sb–Cd–Sb angles vary between 101.95(1)° and 113.36(2)°. These
metric parameters are comparable to the ones reported for other intermetallic
compounds with structures based on CdSb4 tetrahedra. Almost all interatomic distances in the substitution
derivatives Ba3–xAxCd2Sb4 (A = Ca, Sr, Eu, Yb) are systematically shorter than those in
Ba3Cd2Sb4, following the contraction of the
unit cell volume. Notable exceptions are the Sb–Sb bonds, which seem to
elongate upon mixing of the larger Ba2+ with the smaller Ca2+,
Sr2+, Eu2+, and Yb2+ cations.
]
plane and are separated by the large Ba2+
cations (shortest interlayer distance greater than 4.7 ). The Cd–Sb
distances fall in the narrow range from 2.9125(4) to 2.9531(5) and the
Sb–Cd–Sb angles vary between 101.95(1)° and 113.36(2)°. These
metric parameters are comparable to the ones reported for other intermetallic
compounds with structures based on CdSb4 tetrahedra. Almost all interatomic distances in the substitution
derivatives Ba3–xAxCd2Sb4 (A = Ca, Sr, Eu, Yb) are systematically shorter than those in
Ba3Cd2Sb4, following the contraction of the
unit cell volume. Notable exceptions are the Sb–Sb bonds, which seem to
elongate upon mixing of the larger Ba2+ with the smaller Ca2+,
Sr2+, Eu2+, and Yb2+ cations.
The Sb–Sb distance in Ba3Cd2Sb4 is 2.8114(8) , appreciably shorter than the 2.908 contacts in elemental Sb. This is not unusual given that dSb–Sb on the order of 2.8 are well known for compounds with related exo-bonded structural elements, such as Ba11Cd6Sb12, as well as some simpler binaries – KSb and Rb5Sb4. Noteworthy, as mentioned above, is the fact that the Sb–Sb distances are elongated on shortening the unit cell axes – for example, in Ba2.37(1)Sr0.63Cd2Sb4, where the volume change is most significant, ca. –2.5%, the Sb–Sb bonds are 2.8291(8) , almost 0.8% longer compared to the Sb–Sb bonds in Ba3Cd2Sb4. Similar subtlety of the bonding was previously discussed for the pair of isostructural compounds Sr11Cd6Sb12 and Ba11Cd6Sb12, where regardless of the large volume change, dSb–Sb remains virtually unchanged. All of the above is indicative of a very strong covalency of the Sb–Sb interactions.
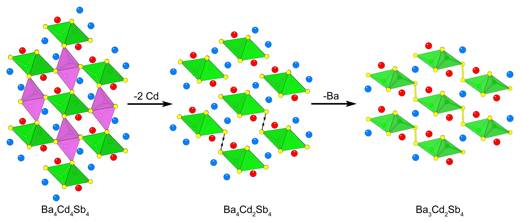
A different description of the Ba3Cd2Sb4
structure can be proposed once its close relationship with the ubiquitous
TiNiSi structure type is recognized. This structure boasts a polyanionic 3D
framework of corner- and edge-shared tetrahedra and cations residing within its
channels. Using an imaginary cutting and pasting, as schematically illustrated
in Figure 6, the structure of Ba3Cd2Sb4 can be
conveniently derived from that of the hypothetical BaCdSb compound (TiNiSi
type), reformulated for convenience as Ba4Cd4Sb4. It requires removing a selected half of the CdSb4 tetrahedra from
the framework, in a way that isolated ![]() [Cd2Sb4]8–
chains of edge-shared tetrahedra are left behind. Next, through a small
distortion, these fragments are brought closer together and joined via adjacent
Sb apexes to form
[Cd2Sb4]8–
chains of edge-shared tetrahedra are left behind. Next, through a small
distortion, these fragments are brought closer together and joined via adjacent
Sb apexes to form ![]() <[Cd2Sb4]6–
layers, with exactly the same topology as the layers in Ba3Cd2Sb4. Finally, due to the formation of Sb–Sb exo-bonds and the changed crystal packing requirements, one of
the Ba2+ cations becomes redundant and needs to be removed. The
outlined imaginary transformations accounts for not only the structure, but
also the composition of the title compound according to the balanced equation:
<[Cd2Sb4]6–
layers, with exactly the same topology as the layers in Ba3Cd2Sb4. Finally, due to the formation of Sb–Sb exo-bonds and the changed crystal packing requirements, one of
the Ba2+ cations becomes redundant and needs to be removed. The
outlined imaginary transformations accounts for not only the structure, but
also the composition of the title compound according to the balanced equation:
Ba3Cd2Sb4 = Ba4Cd4Sb4 – 2«Cd – Ba
Last, following this description of structural relations, and before moving on to a more in-depth analysis of the electronic structure, it is useful to discuss the electron count using the Zintl rules. Following them, Ba3Cd2Sb4 should be a charge-balanced Zintl phase according to the formulation (Ba2+)3[(4b-Cd2–)2(3b-Sb0)2(2b-Sb1–)2].
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6