Reports: G5
46724-G5 Ab Initio Study of the Potential Dependence of the Surface Structure and Reactivity of Doped Ceria Anodes for Use in Direct Hydrocarbon Solid Oxide Fuel Cells
Figure SEQ Figure \* ARABIC 1. Phase stability diagram for single Pd atoms on the CeO2(111) surface. Incorporated Pd atoms are uniquely stable on this surface as surface reconstruction provides for a stable octahedral coordination (illustrated on right).
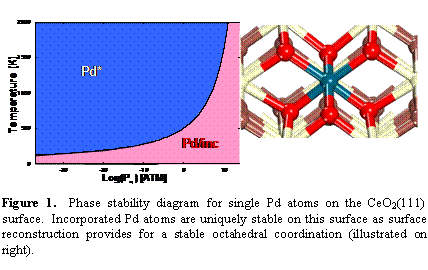
This project utilizes quantum chemical methods to probe the activity and stability of mixed oxides for use as anode electrocatalysts for direct hydrocarbon oxidation in a solid oxide fuel cell (SOFC). The second year of the proposal concentrated on evaluating the energetics of a complete catalytic cycle for methane combustion on ceria and Pd-ceria surfaces as well as a detailed investigation of the stability of single atom states of Pd on various ceria surfaces. The DFT+U method has been used to model the electronic structure of the doped ceria surface, allowing for proper representation of the reduced states. The ab initio thermodynamics approach was used to investigate the relative stability of various single atom states of Pd on different single crystal terminations of ceria. Pd atoms were considered as adsorbed adatoms, adsorbed PdO and PdO2 species, inserted within void spaces within CeO2, and incorporated into Ce lattice positions in the surface of CeO2. For the incorporated states, the formation of oxygen vacancies next to incorporated Pd atoms was also considered. We have demonstrated that single Pd atoms may be stable as supported adatoms or incorporated into the surface of ceria, and that the stable state is dependent on the temperature, oxygen pressure, and ceria surface termination. Figure 1 illustrates the single Pd atom on CeO2(111) phase diagram as well as the structure of Pd incorporated into the surface lattice. The incorporated Pd atom is uniquely stable in the 111 surface because this surface reconstructs to offer the Pd4+, d6 metal atom the stable octahedral coordination environment as predicted by crystal field splitting arguments.
Investigates of the overall combustion cycle of methane illustrate that trends found for the initial methane activation step, reported in year 1, continue throughout the reaction cycle. The CeO2 surface shows high barriers for C-H activation steps that therefore limit the overall rate of oxidation. Contrarily, Pd incorporation into the surface drastically reduces the C-H dissociation activation barrier such that, under solid oxide fuel cell anode conditions, reoxidation of O vacancies limits the overall activity. These results now form the framework for further screening of surface dopants that may be both stable incorporated in the surface of ceria and properly balance C-H bond activation and reoxidation effectiveness. More generally, our work has illustrated that single metal atoms on a reducible support show extreme support interaction effects, and analysis is better considered in the context of mixed-oxide catalysis then supported metal catalysis.
The funding of this ACS-PRF G proposal has enabled my group to initiate this research into rare earth oxide materials for catalysis of energy relevant reactions. This project enabled us to develop an additional capability in the use of the DFT+U methodology. This capability and my developing expertise in the catalytic chemistry of ceria and other rare earth oxides has lead to my inclusion a funded Energy Frontier Research Center, lead out of Louisiana State University including direct collaboration with Kerry Dooley (Chem. Eng., LSU). This project will initiate in Fall 2009 and will investigate mixed rare earth oxides for cleanup of synthesis gas.
Adam D. Mayernick is completing his Ph.D. thesis research on this project under my advisement. The grant funds were used to support his tuition and stipend during the 2008-2009 school year. Adam has developed the ability to perform electronic structure calculations and to apply them to this challenging catalyst problem. Additionally, he has completed coursework in Electrochemical Engineering and is developing an expertise in alternative energy technologies. Adam is first author on the first publication coming from this project. He was recently one of 580 young researchers selected to attend the 59th meeting of Nobel Laureates dedicated to Chemistry. He has also been nominated by our department for fellowship support from Air Products.




