Reports: G1
48479-G1 Synthesis of Chiral Allenyl Ethers: Memory of Chirality in the Cyclization Reactions of Oxyallyl Cations
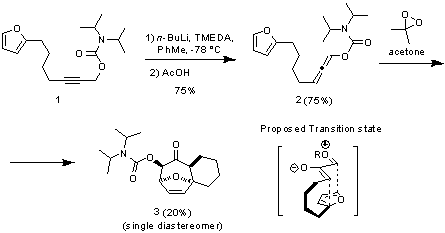
In the first year of funding from the Petroleum Research Fund, we focused upon the inter- and intramolecular cycloadditions of oxyallyl cations generated by the epoxidation of racemic allenyl ethers, with the anticipation of further extending the methodology to asymmetric processes (ie, using chiral allenes) in subsequent studies. The initial investigation of the intermolecular cycloaddition process did not yield the desired cycloadducts. Turning to intramolecular cycloaddition substrates which were readily prepared from simple propargylic carbamates such as 1 led to modest results. Simple epoxidation of allenyl ethers such as 2 resulted in the generation of a reactive oxyallyl cation which underwent a highly diastereoselective cycloaddition reaction to afford the tricyclic compound 3 as a single diastereoisomer. The mild reaction conditions under which the cycloaddition proceeds are encouraging for our future endeavors to render this process asymmetric. Given the ready access to chiral allenyl ethers such as 2 using methodology developed by Hoppe and coworkers, efforts to further improve this intramolecular cycloaddition reaction and its asymmetric variant are currently underway.