Reports: B4
47790-B4 Solution Medium Effects on the Kinetics and Mechanism of Photolysis of Polycyclic Aromatic Hydrocarbons in Aqueous Medium
Introduction
In a previous study funded by an ACS PRF Type G starter grant we measured the photo-oxidation of pyrene in aqueous solution as a function of environmentally relevant solution media, specifically ionic strength and humic acid concentration (Clark et al., 2007). One of the key findings from this work was that photodegradation was inhibited by up to 50% by a commercial humic acid, and that rate constants varied by as much as an order of magnitude over the range of ionic strengths and humic acid concentrations found in natural waters. The observed inhibition was much larger than can be accounted for by simple competitive light absorbance. The inhibition mechanism is unclear, but could result from a reduction in the lifetime of the PAH excited state due to the PAHs binding to DOM. The reduced lifetime would result in a lower degradation rate. Substantial differences have been observed in sorption properties for DOM from different sources, attributed to varying aromaticity, polarity, and molecular weight (Kopinke et al., 2001; references therein). Previous studies on the sorption of PAHs to DOM have also given conflicting results, suggesting both a simple partitioning model (Kopinke et al., 2001) and site-specific interactions (Laor and Rebhun, 2002). An understanding of the binding is needed to assess the impact of DOM on PAH photolysis. In this grant, we proposed extending our analytical methodologies, developed for pyrene and appropriate for undergraduate research, to 5 other common PAHs in natural waters (phenanthrene, anthracene, fluoranthene, chrysene, benzo(a)pyrene). Since DOM conformation (and possibly PAH binding) changes with ionic strength, photolysis rates, mechanisms and sorption processes/binding constants would be examined as a function of ionic strength and humic acid concentrations. A commercial humic acid would be used as a CDOM proxy. Ultimately, these experiments will be extended to a natural water matrix with ambient DOM levels. Four undergraduate chemistry majors worked on this over the last year:
· two seniors on capstone research projects taken as course credit
· a freshman SUMR scholar in summer 2009.
· a junior who supervised the freshman over summer 2009.
Students set up a second
HPLC system for this project and developed GC/MS/SPME and fluorescence binding analytical
protocols for the sorption studies. Photolysis experiments as a function of salt
concentration and Suwannee River humic acid concentration have been
completed. Initial binding experiments
have been carried out for pyrene, phenanthrene, anthracene, benzo anthracene,
fluoranthene, chrysene, and benzo(a)pyrene for both Swanee River Humic acid and
Results to date
Anthracene photolysis:
· A solar simulator was used as an irradiation source to mimic natural sunlight.
· Photolysis followed first-order behavior, with a rate constant of ~0.5 s-1.
· No salt dependence was observed.
· Photolysis rates decreased from 0.5 to 0.1 s-1 at 0.1 mg/L of humic acid. This decrease was due solely to competitive absorption based on model calculations.
Phenanthrene photolysis:
· An unfiltered xenon lamp was used as an irradiation source as no detectable decay was observed with the lower radiation levels of the solar simulator.
· Photolysis followed first order behavior, with a rate constant of ~0.04 s-1 with the lamp (equivalent to ~0.004 s-1 with the solar simulator, or about 100 times slower than observed for anthracene).
· No salt dependence was observed.
· Photolysis rates decreased with humic acid due solely to competitive absorption based on model calculations.
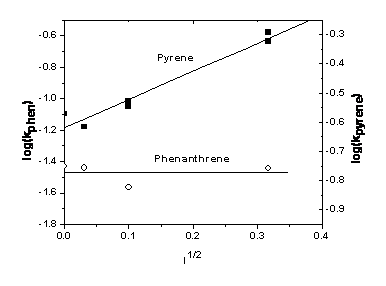
These results for phenathrene and anthracene are in contrast to those we previously obtained for pyrene, which exhibited: 1) a salt effect suggesting the role of charged species in a rate limiting step in the kinetic mechanism; and 2) significant DOM inhibition beyond simple competitive absorption. These results suggest that these PAHs undergo different photodegradation pathways via photoionization (pyrene) and via direct photolysis (phenanthrene and anthracene), and have different binding constants to humic acid.
Figure 1: Photolysis rate constants vs. humic acid concentration. Open circles are experimental data for pyrene, with linked filled squares corresponding to predicted k values based on competitive light absorption by the humic acid.
Figure 2: log of the photolysis rate constants vs. the square root of ionic strength (NaCl solutions)
Binding studies:
For the sorption studies, two methods were tested, GC/MS/SPME and spectrofluorometric. The second was selected based on sensitivity, reproducibility and ease of student use. Preliminary data have been obtained, showing that pyrene has higher binding constants to humic acid than the other compounds. While very preliminary there also appears to be some correlation between PAH structural properties and binding constant..
Future work:
Over the next two years, we plan to:
1. Perform the same experiments on the remaining 3 PAHs proposed
2. Identify photodegradation products using GC/MS and spectrofluorometric methods
3. Complete binding studies for all 5 PAHs in unpurged air-equilabrated solutions as previously done in the literature to compare results, then repeat in purged solutions using a flow through cell to remove potential oxygen artifacts that have not been accounted for in previous studies.
4. Compare to sorption and photodegradation studies in spiked natural water samples with ambient CDOM.