Reports: AC5
47155-AC5 Molecular Adsorption on Metallic Nanostructures: Tailoring Chemisorption by Quantum Confinement of Electrons
The goal of this research is to identify how quantum size effects in nanoscale metal structures impact their interaction with small molecules. In this funding period, we had two major accomplishments. First was to determine the chemisorption properties of small molecules on the metastable fccCo(100) surface. Secondly we investigated CO adsorption on the metastable Cu/fccFe/Cu(100) system using infrared spectroscopy and observed that the coupling between C-O stretch vibrational modes of CO at different adsorption sites appears to be modulated by quantum size effects.
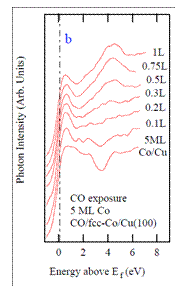
There are a number of way in which metallic quantum well states (MQWs) may influence the adsorption of small molecules. In some cases the density of states at the Fermi level is modulated as MQW states move through EF with increasing film thickness. Another possibility is a direct interaction between MQW states and the energy levels of the adsorbed molecule. To investigate this latter possibility, we explored the adsorption properties of CO on metastable ultrathin fccCo films as a function of film thickness. Inverse photoemission spectroscopy (IPS) data from clean and CO-covered epitaxial fccCo/Cu(100) are shown in Fig 1. MQW states are seen between ~1 eV and ~ 3 eV above the Fermi level, an energy range near the unoccupied CO 2p* antibonding level. The CO 2p* level has little intensity for exposures less than 0.3 L, but then shows little change in the energy or lineshape with increasing CO exposure.
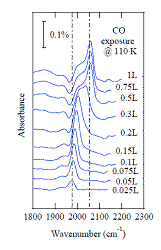
Infrared spectroscopy indicates that CO adsorption on this surface is more complicated than the IPS results might suggest. Fig. 2 shows a series of FTIR spectra in the region of the CO stretch vibration for the 5 ML Co/Cu(100) MQW system as a function of increasing CO exposure. For the lowest exposures, the spectrum is dominated by a peak at 1974 cm-1 and a small shoulder at 1936 cm-1. As the CO exposure increases through 0.15 L, the intensity of the high frequency peak increases and its frequency shifts to ~ 1998 cm-1 in a monotonic fashion. At an exposure of 0.2 L, the main peak has shifted to 2005 cm-1 and becomes asymmetric with intensity extending to 2025 cm-1. By an exposure of 0.3 L, the absorption feature becomes broad and extends to higher wave numbers, having a well-defined peak at 2038 cm-1 and a shoulder with a local maximum near 2000 cm-1 that appears to contain several overlapping features. At exposures of 0.5 L and higher, the spectrum is dominated by a peak at 2060 cm-1 with a broad shoulder extending from ~ 2000 cm-1 to ~ 2030 cm-1.
From these data we arrive at the following model for
adsorption of CO on the fcc-Co(100) surface at low temperatures: At low
exposure, adsorption is primarily on the atop site, although a small fraction
of bridge bonding is also present. With increased exposure up to ~ 0.2 L, additional CO molecules are
primarily accommodated at atop sites and are in close proximity giving rise to
the dipole shift. For low temperature
exposures greater than 0.2 L, weakly bound configurations form at the surface,
as indicated by the presence of higher frequency CO stretch modes. These
molecules presumably bind at non-ideal sites, perhaps near the boundary of any
small adsorbate islands. At higher exposures (> 0.5 L) the
mode at 2060 cm-1 is very close to the calculated stretch
frequency of the Co(CO)2 molecule, suggesting that a subset of the
CO molecules may
enter a compressed phase where more-than-one CO is bound to a given Co
surface atom. For low temperature
adsorption on the hcp-Co(10-10) surface, a compressed phase on the terrace,
where a higher surface concentration of molecules is accommodated by tilting
the axis of the molecule away from the surface normal, is thought to give rise
to a ~ 20 cm-1
shift to higher frequency, from 1967 cm-1 to 1984 cm-1.
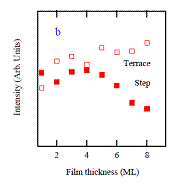
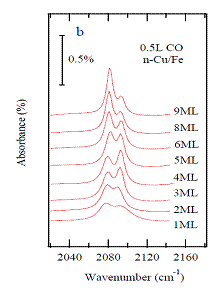
Figure 3 shows FTIR spectra of 0.5 L CO covered Cu(fccFe/Cu(100) system
for copper films of different thicknesses.
Two features are observed: at 2080 cm-1, and at 2095 cm-1.
From measurements on the single crystal Cu(100) surface, the lower frequency
peak is associated with terminally bonded CO and the higher frequency peak with
CO bonded to step or defect sites. Also
consistent with the literature is that although the step or defect atoms
constitute < 10% of the surface, they account for as much as ~ 50% of the
spectral weight.
The unexpected result found in Fig. 3 is that the relative terrace and step site intensities change as a function of Cu film thickness. The results of a Lorentzian fit of the spectra as a function of film thickness is shown in Figure 4. For the specific thickness of 4ML Cu, the step-site intensity reaches its maximum and then diminishes with further increase in thickness. This is also the thickness where the first MQW state crosses the Fermi level. STM measurements showing similar step and defect densities for all film thicknesses studies rules out the possibility that this variation is due to surface thickness-dependant changes in morphology.
From the analysis given above it is very suggestive that by modulating electronic structure, QSEs play an important role both in oscillating changes of TPD peak Td (as found in our earlier work) and in bonding site absorbance intensities of FTIR spectra. The specifics of how QSEs modify step and terrace absorbance intensities remain unclear and a more systematic study of the CO/Cu/fcc-Fe/Cu(100) system is required. This will be a future direction of the research.