Reports: AC4
47103-AC4 New Model Systems to Measure Weak Non-covalent Interactions
The goal of the project is to design and test small molecule systems to measure weak non-covalent interactions in order to better understand the variables that influence their stability. Non-covalent interactions such as hydrogen bonds and arene-arene interactions are important in determining the folding of proteins, the specificity of biologically active molecules, and in the selectivity of organic transformations. A better understanding of the strengths of non-covalent interactions would allow us to more accurately predict and design better systems for the above applications.
In the first reporting period, we developed a new molecular torsional balance (shown below) to measure and study face-to-face arene-arene interactions. In this second reporting period, we focused to specific goals: 1) applying this new molecular balance to study the electrostatic and solvent dependence of the face-to-face arene-arene interaction and 2) developing new versions of the balance to study other non-covalent interactions of arene surfaces.
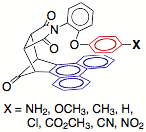
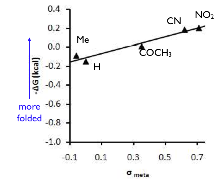
For the first goal, we synthesized a series of balances with arene surfaces of varying electrostatic potential by varying the para-substituent (X-group) on the arene arm. A strong electrostatic effect was observed in which more electron-poor arene surfaces made a proportionally stronger arene-arene interaction than electron-rich arene surfaces. This electrostatic attraction was also strongly attenuated in different solvents. Specifically, we found that solvents that were good hydrogen bond donors were the best at disrupting the electrostatic attraction of the arene surfaces. More recently, we have also been examining the effects of solvation on the arene-arene interaction and we found that there was a surprisingly strong solvophobic effect in organic solvents that drives the arene surfaces together. The solvophobic effect increases with increasing solvent polarity as measured by the ET(30) solvent polarity parameter as shown in the plot below. This has important consequences for the design of catalysts and reactions that are carried out in organic solvents that rely on arene-arene interactions for rate acceleration or selectivity.
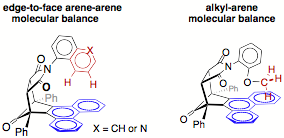
For the second goal, we have developed a parallel series of molecular balances to measure other arene-arene interactions such as edge-to-face arene-arene interaction in which the two arene surfaces are oriented 90 degrees with respect to each other and alkyl-arene interactions between weakly polarized C-H bonds and arene surfaces. The structure below on the left is an example of our edge-to-face arene-arene balances. The two hydrogens on the edge of the naphthalene or quinoline rings form interactions with the phenanthrene surface below. This was confirmed by x-ray crystal structures and by NMR studies. Similarly, the structure below on the left is an example of our alkyl-arene balance. Again, we have confirmed the formation of an attractive interaction between the highlighted protons on the methyl group and the opposing phenanthrene surface.
In conclusion, we have developed a very simple small molecule system in which we can now accurately measure and study a wide range of non-covalent interactions. Using the research results and data from this PRF funded project we were able to garner funding for a four year grant from the NSF entitled, "A molecular balance for studying the non-covalent interactions of aromatic surfaces". We are very appreciative of the PRF for providing funds that seeded the development of this project and this new research direction in our group.