Reports: G10
48158-G10 Catalyst Engineering Using Suport-Dopant Modification: Towards Intelligent Design of Catalyst Systems
This project investigates the use of nitrogen-doped carbon supports to improve the catalytic activity of various low temperature electrochemical reactions relevant to fuel cells. Recent research suggests that catalytic activity for both methanol electrooxidation and oxygen reduction is dramatically improved when Pt catalysts are loaded onto nitrogen-containing carbon supports vs. pure-carbon supports [[i],[ii],[iii]].This effect is so significant that it could be game-changing for fuel cell catalysis. The principle goal of this research effort is to determine why nitrogen-doping improves catalytic activity. The project employs highly controlled, geometrically simple Pt/graphite test-systems to clearly measure and explain nitrogen-doping effects on catalytic activity. In particular, this approach will determine whether structure, chemistry, or a combination of effects leads to the nitrogen-doping enhancement.
To investigate the fundamental reasons underlying N-doping enhancement, we have developed geometrically well-defined model catalytic systems consisting of assemblies of Pt catalyst nanoparticles arrayed on clean, planar substrates of N-doped, Ar-doped, and undoped highly-oriented pyrolytic graphite (HOPG). Contrasting the N-doped samples to the undoped and Ar-doped samples provides two sets of control comparisons. The unmodified samples provide a pristine control, while the Ar-doped samples represent an inert dopant species control. Our primary investigation techniques have so far included SEM, AFM, Raman, XPS, and cyclic voltammetry (to assess catalytic activity). Our results strongly support the theory that doping nitrogen into a graphite support significantly affects both the morphology and behavior of the overlying Pt nanoparticles. In particular, N-doping is observed to cause a decrease in Pt nanoparticle size, an increase in nanoparticle dispersion, and an increase in catalytic activity and durability for a variety of fuel cell reactions. (See figures and figure captions, below)
Figure 1: Current catalyst systems (a) compared to an envisioned dopant engineered catalyst system (b). Dopant engineering may lead to a more homogeneous, durable, active, and selective catalyst system. The goal of this research effort is to determine whether the dopant engineered system envisioned in (b) is feasible.
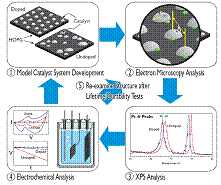
Figure 2: Schematic diagram illustrating our approach using model HOPG/catalyst systems to investigate N-doping effects. 1) Both N-doped and undoped model catalyst systems are created. 2) Microscopy is used to quantify structural features (such as catalyst particle size, density, distribution). 3) XPS analysis is used to quantify chemical effects of the N-doping. 4) Electrochemical experiments are used to quantify catalytic activity and durability. 5) The catalysts are re-examined after electrochemical durability testing to quantify structural degradation mechanisms.
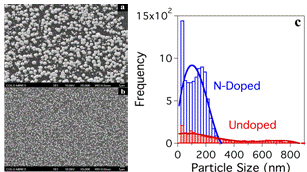
Figure 3: Results from our well defined catalyst systems confirm the N-doping effect, proving that nitrogen-doping yields dramatic decreases in the size of supported Pt nanoparticle catalysts: (a) SEM image of Pt nanoparticles grown on an undoped HOPG substrate vs (b) a N-doped substrate (identical growth conditions, same magnification). (c) Pt particle size distribution comparison between the doped and undoped samples. The N-doped sample yields significantly smaller average particle size and tighter particle size distribution.
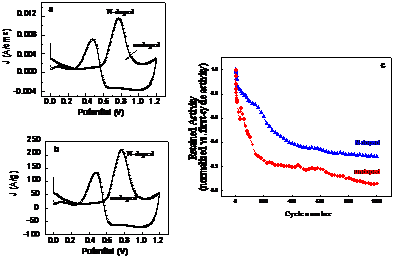
Figure 4: Nitrogen-doping yields dramatic enhancements in activity and stability of carbon-supported Pt nanoparticle catalysts: (a) Mass-normalized methanol oxidation reaction (MOR) activity comparison for N-doped vs. undoped sample. The N-doped sample shows ~9X greater mass activity. (b) Pt-surface area normalized MOR activity comparison. The N-doped sample shows ~3X greater intrinsic activity per unit Pt surface area. (c) Cycling degradation resistance comparison of the N-doped and undoped samples. After 1000 cycles, the N-doped sample retains ~30% of its initial activity. In contrast, the undoped sample retains only ~6% of its initial activity. Experimental details in [[iv]].
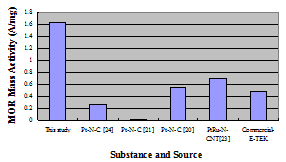
Figure 5: Our N-doped catalyst obtains among the highest CV catalyst mass activity values recorded for the methanol oxidation reaction (MOR).
This young investigator's grant from the ACS PRF has been instrumental in providing seed funding for my initial results in this area. This grant has yielded early results which have enabled me to seek additional research funding from government sources. In particular, partly as a result of this work, I have just been awarded the Presidential Early Career Award in Science and Engineering (PECASE). The budget flexibility provided by this ACS PRF grant has been particularly important, as I've required adjustments based on staffing situations and a capital equipment acquisition opportunity which occurred during the year. Specifically, budgeted graduate student salary/tuition expenses for the 08/09 year were not incurred because the graduate student I had recruited decided to work on another project instead. I have bootstrapped the work on this project primarily through my own time and through the pro-bono efforts of a post-doc who is currently supported on another grant. In the meantime, I have used the ACS grant funds to leverage a DoD equipment grant to purchase a combined scanning confocal-Raman/atomic force microscopy instrument. This instrument enables my lab to characterize both the structural and chemical effects of nitrogen doping for this project. The results from this instrument purchase have already been featured heavily in several of our accepted/submitted research papers over the past year. The microscope acquisition would not have been possible without the ACS grant funds. In the upcoming year, a new graduate student will be assigned full time to this project and will be partially supported by ACS funds.
[[i]] T. Maiyalagan, B. Viswanathan, U.V. Varadaraju, Nitrogen-containing carbon nanotubes as supports for Pt-alternate anodes for fuel cell applications, Electrochem. Comm., 7, 905-912 (2005)
[[ii]] S. Ye, A.K. Vijh, L.H. Dao, A new fuel cell electrocatalyst based on carbonized polyacrylonitrile foam: the nature of platinum-support interactions, J. Electrochem. Soc., 144(1), 90-95 (1997)