Reports: GB3
47598-GB3 Chelation Directed C-H Functionalization Reactions with Ruthenium Boryl Complexes
Directed C-H functionalization (carbon-hydrogen bond to carbon-heteroatom bond) is becoming a valuable tool in organic synthesis. Applications of this methodology, however, are currently limited by the carbon-heteroatom bond that can be formed. C-H functionalization with metal boryl complexes provides regioselective formation of carbon-boron bonds (selective for aryl and terminal alkyl C-H bonds) from carbon-hydrogen bonds. Because the C-B bond can be converted into C-C, C-O, C-N, and other C-X bonds, this method provides a very general way to convert simple substrates into synthetically valuable products if a single reactive site is present in the substrate (for example, one terminal CH3 group).
This report describes our progress in the synthesis and evaluation of the reactivity of boron-substituted analogues of the Shvo hydrogenation catalyst (1, Scheme 1). Initial results in this area focused on optimizing the synthesis of ruthenium boryl complexes and the exploration of the resulting reactivity of these complexes in hydroboration and CH functionalization reactions. Replacement of the ligand-based OH group with an OB group (2) has led to a complex that is reactive in the hydroboration of aldehydes, ketones, and imines (published in Organometallics, 2009). Replacement of the metal-based RuH with a RuB substituent (3) results in a complex that functionalizes C-H bonds alpha- to oxygen of an ether substrate selectively over alternative terminal C-H bonds. The resulting boronate esters are prone to decomposition. Current efforts are focused on finding conditions that lead to isolation of these valuable products.
Scheme 1. Boron-Substituted Analogues of the Shvo Hydrogenation Catalyst
Scheme 2. Synthesis of RuHOBpin 5
Scheme 3. Synthesis of Ruthenium Boryl Complexes
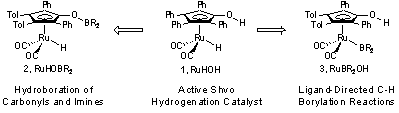
The initial focus of our research was
on the synthesis and reactivity of RuHOBR2 (2, Scheme 1). Synthesis of this complex was achieved by addition of
pinacolborane to ruthenium dimer 4
(Scheme 2). The resulting complex (RuHOBpin 5) was found to be catalytically active in the hydroboration of
aldehydes, ketones, and imines. Preliminary mechanistic studies suggest that
the reaction proceeds by a similar pathway to the unusual outer-sphere
ligandmetal bifunctional Shvo catalyst (1,
Scheme 1). See publication for details.

Synthesis of ruthenium boryl complexes (such as 3, Scheme 1) was achieved by the
activation of bis(catecholato)diboron in the presence
and absence of a phenol. The addition of bis(catecholato)diboron
to ruthenium dimer 4 provided RuBcatOBcat complex 6
in 70% NMR yield (Scheme 3). The OB bond of complex 6 was highly labile, precluding the isolation of the pure complex.
Addition of 4-methoxyphenol to complex 6
resulted in selective cleavage of the OB bond to generate RuBcatOH complex 7.
Under these conditions complex 7 was
isolated in 39% yield. Alternatively, conducting the reaction between dimer 4 and bis(catecholato)diboron
in the presence of 4-methoxyphenol provided complex 7 in 74% NMR yield. Isolation of complex 7 from this reaction provided a 30% yield. An X-ray crystal structure
of complex 7 confirmed the
anticipated structure and regiochemistry.
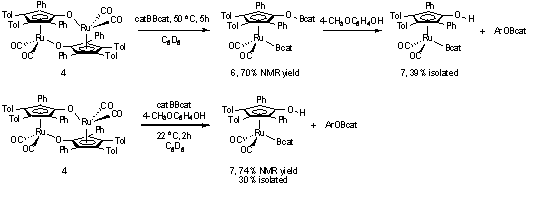
With a method to synthesize ruthenium
boryl complex 7, we investigated the
ability of this complex to mediate ligand-directed CH borylation reactions. The
hydroxy group on the Cp′ ligand was expected to interact with the heteroatom of a substrate through a hydrogen
bond, resulting in directed functionalization of the C-H bond via an optimized transition-state structure.
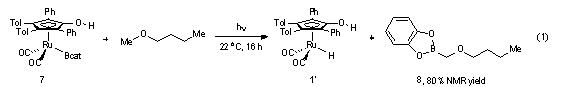
Initial development of directed C-H borylation reactions was examined with simple ether substrates.
Ruthenium boryl complex 7 was
photolyzed in the presence of diethyl ether-d10,
providing several undesired products. The poor selectivity of diethyl ether-d10 likely reflects a
non-optimal chain length for C-H
functionalization. Butyl methyl ether was chosen as the next substrate due to
the presence of multiple potentially reactive C-H bonds (eq 1). Under similar photolytic conditions,
butyl methyl ether provided an 80% NMR yield of 8 (> 95% selectivity by 1H and 11B NMR
spectroscopy), resulting from functionalization of the methoxy CH bond. This
result is consistent with an optimal transition-state structure for directed C-H functionalization.
a-Alkoxy boronate esters are known to be prone to
decomposition. Isolation of these substrates by the exclusion of air and
moisture is typically possible. In our experience, boronate ester 8 is difficult to separate from the
ruthenium hydride (1') by-product
without decomposition. Attempts to filter out 1' or distill off 8 have
been unsuccessful, typically resulting in multiple decomposition products. We
are currently attempting to overcome this isolation issue by derivatizing 8
into an air- and moisture-stable product (Scheme 4). Examples of a-alkoxy trifluoroborate salts, for example,
were reported by Molander to be stable products.
Synthesis of trifluoroborate salt 9
was achieved by a procedure reported by Molander for
related substrates. We are currently developing conditions for the conversion
of 8 to 9.
Scheme 4. Conversion of Boronate Ester 8 to Trifluoroborate Salt 9

|
In addition to the results described above, the synthesis of additional ruthenium boryl complexes is being explored. The activation of B2cat2 shown in Scheme 3 provides low yields for the activation of B2pin2. The application of pinacolato-substituted boronate esters to ligand-directed CH borylation reactions is desired because these complexes typically provide higher yields in the CH functionalization reaction and also provide products that are less prone to decomposition. Toward this end, we have begun to explore the synthesis of boron-substituted analogues of Noyori's hydrogenation catalyst (Scheme 5). Treatment of ruthenium chloride 10 with bis(pinacolato)diboron and NaOt-Bu in benzene-d6 resulted in a product with spectroscopic properties that are consistent with the anticipated ruthenium boryl complex 11. Attempts to grow X-ray quality crystals have not been successful up to this point.
Scheme 5. Synthesis of Alternative Ruthenium Boryl Complex 11

|
In summary, the synthesis of boron-substituted analogues of the Shvo hydrogenation catalyst have provided complexes that are active as either hydroboration catalysts or CH borylation complexes. Current efforts are focused on developing a method to isolate CH borylation product 8 and on the synthesis of alternative ruthenium boryl complexes.




