Reports: G10
48227-G10 A Systematic Study on Vapor-liquid-solid (VLS) Growth Process of Metal Oxide Nanodendrites
During the past year, we have conducted the following investigations on the ‘vapor phase growth process of metal oxide nanodendrites:
(I) Synthesis and self-assembly of ZnO nanodendrites with and without involving metal catalyst;
(II) Temperature/pressure dependence study of ZnO nanodendrites growth;
(III) Intermediate metal catalyst effect on the growth process and reaction chemistry of metal oxide nanostructures;
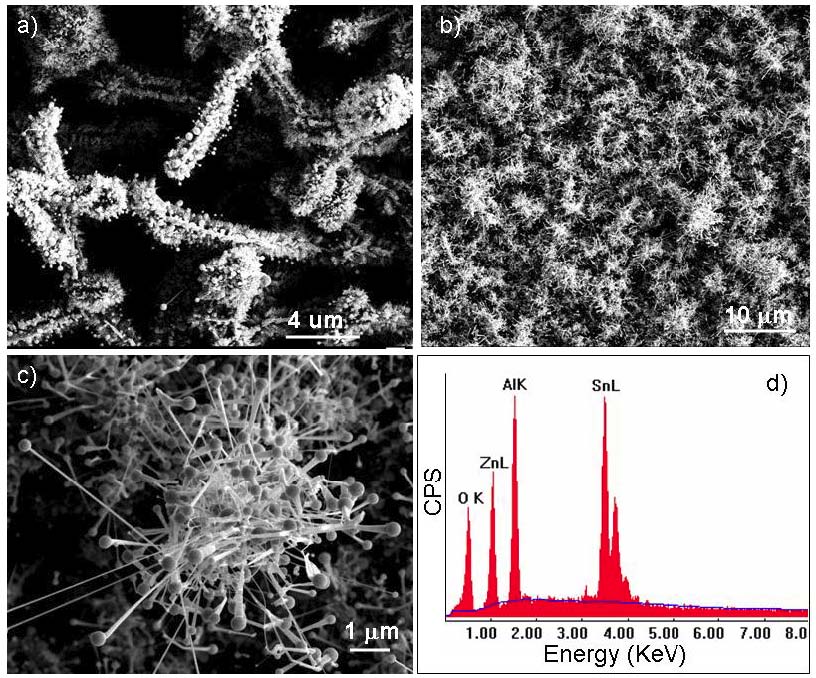
During the synthesis/assembly and temperature/pressure dependence study, we have successfully fabricated large scale Sn catalyzed ZnO nanodendrites using much lower temperature (~900 oC) than the previous studies (~1100 oC-1400 oC). Figure 1a is a typical low magnification scanning electron microscopy (SEM) image of free standing Sn-catalyzed ZnO nanodendrites grown after the ~900 oC vapor phase deposition process involving powders of ZnO, SnO, and C. ZnO nanodendrite arrays can also be achieved at ~900 oC on polycrystalline Al2O3 substrate, as shown in Figure 1b. While a closer look at individual nanodendrites, as indicated in Figure 1c, suggests that the grown nanodendrites are of relatively irregular shape compared to previous study at higher temperature. The BET surface area testing has indicated that the free-standing nanodendrites fabricated here has a tunable specific surface area of ~50-200 m2/g, while the nanodendrite arrays on Al2O3 substrates have variable surface area ranging from 5-50 m2/g depending on the nanowire/belt density, dimensionality, and spacing distribution. Energy dispersive X-ray spectrum (EDXS) spectrum in Figure 1d identified the content of Sn, Zn, Al, and O on the ZnO nanodendrite coated Al2O3 substrate.
Figure 1 a) Free standing ZnO nanodendrites catalyzed by Sn at ~900 oC. b) ZnO nanodendrite arrays grown on Al2O3 polycrystalline substrate. c) A zoom-in SEM image of individual ZnO nanodendrite in b). d) Energy dispersive X-ray spectrum (EDXS) showing the content of Zn, Sn, Al, and O for the ZnO nanodendrites on Al2O3 substrate.
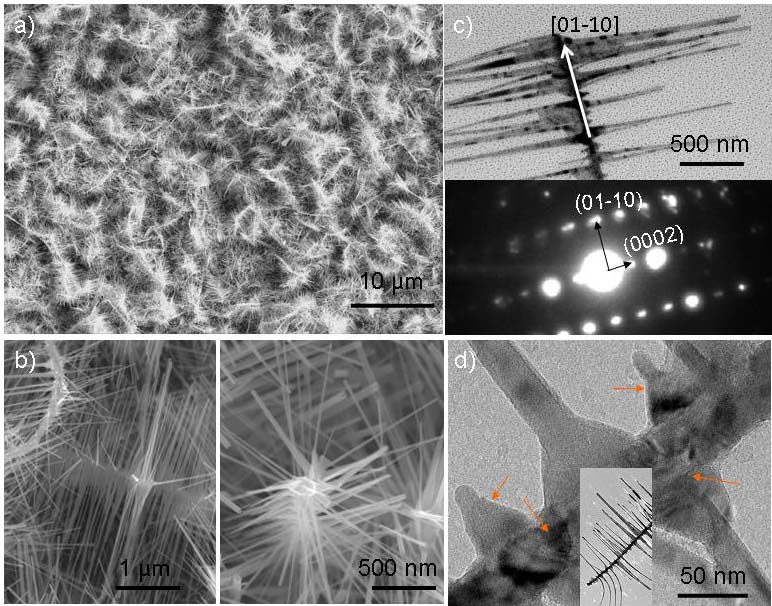
To study the intrinsic crystallography influence of the formation of ZnO nanodendrites, which are a single piece of crystal, ZnO nanofilm have been used for in-situ ZnO nanodendrite growth at relatively low temperature (~800 oC), facilitated by graphite. Two types of pure ZnO nanodendrites have been found (Figure 2a): single crystal nanodendrites and nanocombs (Figure 2c), and multi-crystal nanodendrites and nanocombs (Figure 2d). The core-branch crystal symmetry could be observed in the left zoom-in SEM image in Figure 2c, while the right-side nanodendrite does not exhibit core-branch crystal symmetry, which might indicate the multi-crystal nature of this dendrite structure. It is suggested that different kinetic growth processes might be necessary for differentiating these two types of nanodendrites. It is also suggested that in the Sn catalyzed VLS process, these two types of growth may be found at relatively low temperature region as well.
Figure 2 a) self-catalyzed ZnO nanodendrites grown on Si substrate at ~800 oC. b) ZnO nanodendrites and nanocombs with (left) and without (right) exhibiting certain core-branch crystal symmetry. c) A transmission electron microscopy (TEM) image showing a nanocomb with single crystal structure, inset is the corresponding selected area electron diffraction pattern. d) A high resolution TEM image showing the irregular shaped dendrite branches with distinct diffraction contrast with the core, suggesting the multi-crystal nature.
To investigate the intermediate metal catalyst effect, Ag coated Si substrate has bee used to growth nanodendrites. Also to lower the growth temperature, metal Zn and Sn has been used as the sources materials. A low temperature range of ~400-750 oC has been investigated; no successful growth of ZnO nanodendrites has been achieved. However, large scale ternary oxide Zn2SnO4 (ZTO) single crystalline periodic nanowires have been successfully grown at ~650 oC. A detailed investigation of the behind mechanism has been conducted.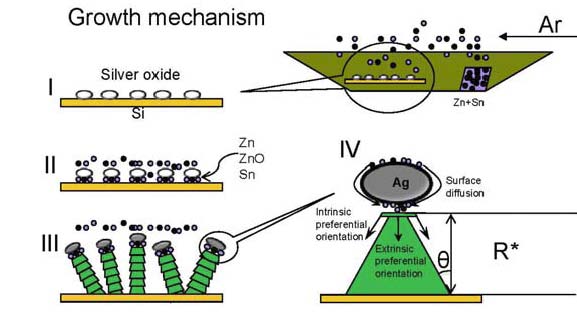
Figure 3 A growth mechanism schematic of the 4-step nucleation and growth model revealing the possible oxide-assisted VSS growth process of ZTO periodic nanowires.
Zn and Sn have relatively low melting points ~419oC and ~232oC, respectively. They can be easily vaporized at ~650oC under a pressure of ~40 mbar. Therefore, Zn and Sn source atoms vaporized and attached to the Ag2O coated substrate surface to which they would attach (Figures 3 I, II). The EDXS result (Figure 4d) confirmed the formation of cubic ZTO nanowires. This suggests the attached Zn and Sn atoms should use O extracted from Ag2O to form ZTO, and leave metallic Ag inside the ball tips. Thereof, the nucleation is suggested to happen in accordance with the following formula (1)×2+(2)=(3). Ag2O (s) + Zn (g) Æ 2Ag (s) +ZnO (s) (1)
2ZnO (s) + Sn (g) + O2 (g)Æ Zn2SnO4 (s) (2)
2Ag2O (s) + 2Zn (g) + Sn (g) + O2 (g) Æ Zn2SnO4 (s) + 4Ag (s) (3)
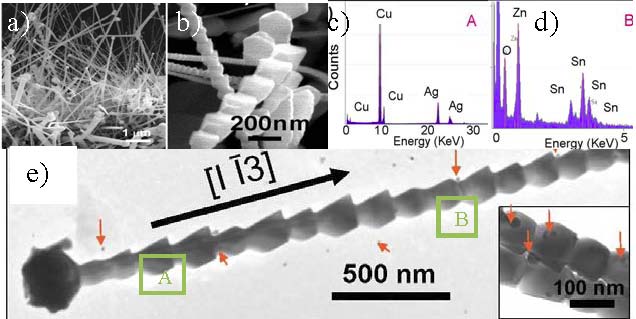
Figure 4. a) Cross-section view of Zn2SnO4 periodic nanowires; b) Closer view of periodic structured ZTO nanowires; c) and d) EDXS results under the TEM (Figure e)) corresponding to tip region A (left spectrum) and nanowire body region B (right spectrum); and e) A low magnification TEM image showing a single ZTO periodic nanowire ~100 nm wide, inset is a enlarged picture of each unit block.
In short, the growth of single crystalline Zn2SnO4 periodic nanowires has been identified to be a unique silver oxide catalyzed VSS growth process. These Zn2SnO4 nanowires mainly grew along [1-13] direction with three major morphologies including straight line, L-shape, and radiative
sparse growth. A two-dimensional nucleation and periodic ledge growth model was used to explain the formation and periodicity of Zn2SnO4 nanowires, as illustrated in Figure 3. Furthermore, these Zn2SnO4 nanowires were found to be sensitive to ~150 ppm ethanol pulses at ~150oC in both close and open environments.References
- P.X. Gao, “Metal-catalyzed nanoarchitectures of zinc oxide and their applications,” Chapter in Metal Oxide Nanostructures and Applications, Ahmad Umar Eds, American Scientific Publishers, Stevenson Ranch, CA, in press, 2009.
- P. Shimpi, Kuo-ting Liao, and P.X. Gao, “A Universal Growth Process for Various Functional Nanowire Architectures Without Using Metal Catalysts,” Materials Research Society Fall meeting, Boston, December, 2009. (Accepted).
- W.J. Cai, P. Shimpi, D.L. Jian, and P.X. Gao, “Oxide catalyzed Ag2O/Zn2SnO4 hybrid nanowires and their reversible catalytic ambient ethanol/oxygen detection,” submitted, 2009.