Reports: AC5
48526-AC5 Click-On SBA-15: Efficient Variable Loading of Oxidation Catalysts in Porous Silica
Surface modification of silica materials with organosilane groups holds considerable potential for many applications, including heterogenization of discrete metal catalysts. Beyond recycling and separation advantages, heterogenized discrete metal catalysts allow for modifications of the electronic and steric demands of the catalyst through modular variations in the organic ligand, a level of control not currently available in heterogeneous metal catalysts. Heterogenization by covalent attachment also allows for potential site-isolation of the catalysts. Relative to its homogeneous counterpart, potential enhanced catalytic activity is possible as deactivation pathways such as catalyst aggregation or oxidative canabalism can be significantly attenuated. Simple and robust synthetic procedures that allow for defined loadings of any covalently immobilized catalyst will facilitate screening of discrete catalysts. Mesoporous silicas are intriguing supports due to their well-ordered structures, large surface areas, and large, well-defined pore diameters. Unfortunately, control over the distribution and loading of organic entities within mesoporous silica has been difficult to achieve through traditional hydrolytic grafting procedures, as the tris-alkoxyorganosilianes generally cluster on the surface when less than a full monolayer is desired.
In our research, hybrid mesoporous SBA-15 silicas were synthesized by a direct synthesis methodology, which allows for predictable azidopropyl loadings (0.03-0.7 mmol g–1) at site-isolated to site-dense coverages (ca. 2-50% surface coverage) in which the azidopropyl groups are dispersed randomly. At the described loadings above, the hybrid silica materials retain the favorable physical attributes of the parent SBA-15 and allow subsequent covalent attachment of ethynylated organic moieties through a copper catalyzed [3 + 2] Huisgen cycloaddition reaction. This chemical reaction proved to be highly efficient within the materials and is now readily followed by simple IR spectroscopy methodology, which was developed for this investigation (Figure 1). Four different examples at variable loadings have been prepared and characterized: ferrocene (SBA-15-Fc-x), pyrene (SBA-15-Pyrene-x), tris(pyridylmethyl)amine (SBA-15-TPA-x), and an iron porphyrin (SBA-15-FeTPP-x), x = loading level. The diversity of chemical group that have been attached and the consistency with which these material can be produced demonstrate that this synthetic attachment strategy is robust and efficient.
|
|
|
|
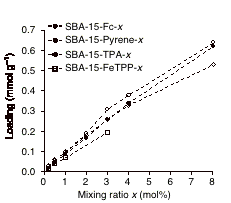
Figure 1. Loadings of amounts (mmol g–1) of SBA-15-R-x (R: Fc, Pyrene, TPA and FeTPP) as a function of the mixing ratio x of Si-N3. Fc and FeTPP loadings were determined by ICP Fe analysis of digested samples. Pyrene and TPA loadings were determined by UV-vis spectrometry of digested samples. |
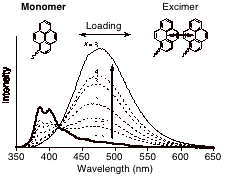
Figure 2. Fluorescence spectra of SBA-15-Pyrene-x (x = 0.2, 0.5, 1, 2, 3, 4, 8 mol%) suspended in CHCl3 (lex = 330 nm). For comparison, the spectra were normalized at 415 nm. |
The pyrene materials at variable loading provide a probe as to the distribution of the original organoazides through the existence/absence of dimers; the dimers fluoresce at much lower energy than the monomers. The SBA-15-pyrene-x materials show clear dependence on loading level (Figure 2), which is consistent with a random distribution of pyrene moieties, and thus the original organoazides. Spectroscopically measurable site-isolation of the attached pyrene with a tether length ca. 15 is achieved in the SBA-15 materials at 1% loading, ca. 0.02 mmol g–1. This level of loading for site-isolation should be applicable to other metal complexes.
The copper(I)-loaded TPA materials, SBA-15-[CuI(TPA)]-x, exhibit dramatic color changes upon reaction with dioxygen at low temperatures. The color change is very dependent on the surface density of the complexes. At high loading, the formed purple color is consistent with the formation of a dimeric copper-peroxo complex, which had been extensively characterized in a homogeneous solution. Site-isolation leads to a persistent green color, identifiable as a 1:1 Cu-dioxygen species, illustrating site-isolation of the complex and the potential advantage of site-isolation in generating oxidized species that have significantly longer lifetimes than in a homogeneous solution.
Demonstration of catalytic reactivity at a metal center has been achieved through the SBA-15-FeTPP-x materials. In homogeneous solutions, iron (III) porphyrins (FeTPP) are known to catalyze amine alkylation efficiently using ethyl diazoacetate, presumably proceeding through a carbene insertion reaction. Heterogenization of the FeTPP provides catalysts that operate with similar efficiencies at all levels of loadings, albeit at slightly slower reaction rates (2X) as anticipated. The comparable reaction rates support the notion that the immobilized catalysts both on the exterior of the particules and within the pores are operative.
The direct synthesis of azidopropyl mesoporous silicas provides predefined loadings of randomly distributed functionalities within the materials, ranging from site-isolated to site-dense. Such control over loadings, along with simply implemented analytic procedures, should facilitate the translation of homogeneous chemistries to heterogeneous supports. A paper covering the full characterization of these materials is currently in revision and will acknowledge the support of the PRF.