Reports: AC3
46828-AC3 Dehydrogenation Catalysis Relevant to Biomass Conversion to Chemicals and Fuels
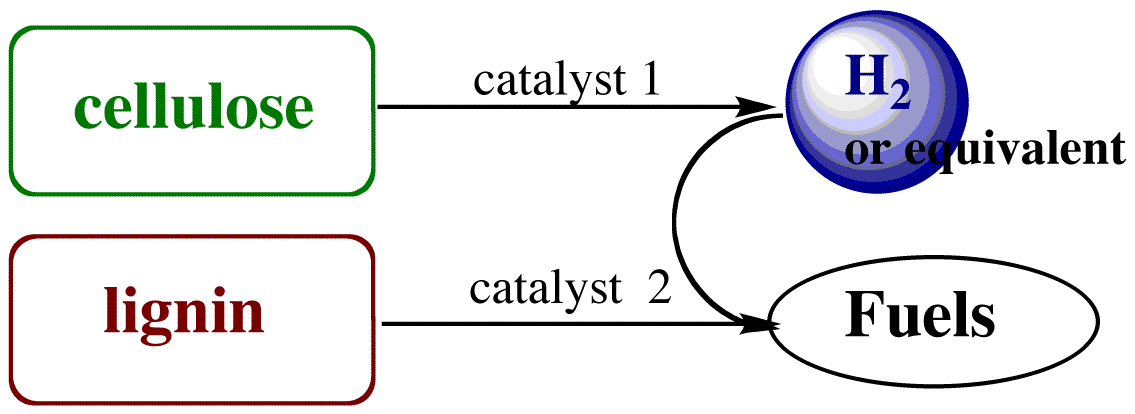
This project is concerned with the development of new catalysts for the dehydrogenation of alcohols and with elucidating principles defining such processes for polyfunctional alcohols that serve as biomass models. The longer-term goal is to utilize such catalytic systems for the degradation of biomass components to dihydrogen (or dihydrogen equivalents). Of particular interest is the development of new methodologies for the transfer of hydrogen equivalents from carbohydrates (including cellulose) to other substrates such as lignin (Scheme 1). With environmental challenges already apparent for fossil hydrocarbons, delineating catalytic strategies for the utilization of carbon-neutral alternative feedstocks is clearly essential to sustained economic health. Materials formed by photosynthesis are CO2 neutral and represent net solar energy storage, excluding the fossil fuels used in their production, harvesting and transportation. Thus, effective and efficient catalytic conversion of non-food biomass to transportation fuels or chemical precursors would be an important addition to the world's energy and supply portfolio.
Scheme 1:
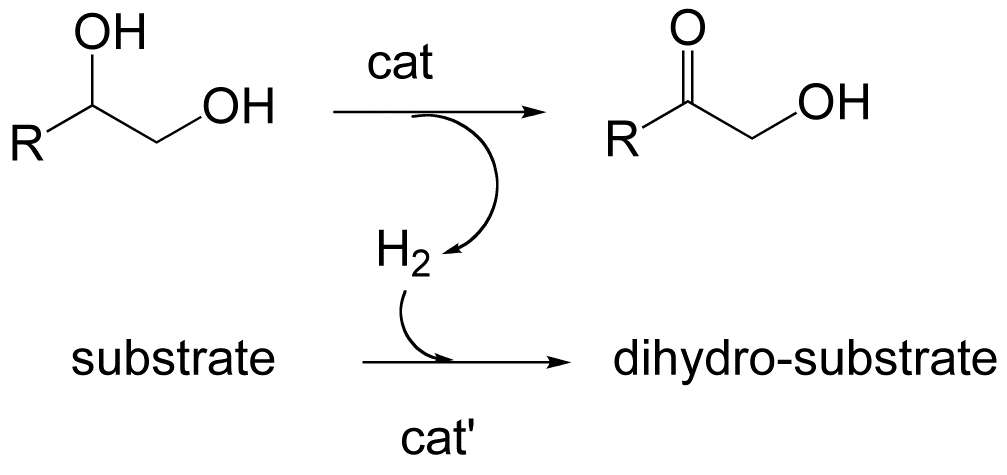
The focus of our ongoing studies proposed is the investigation of chemical transformations that are models for key steps in the multiple catalytic cycles necessary for the degradation of a polyfunctional substrate such as glucose, glycerol or other carbohydrates. Having better mechanistic understanding of operating catalyst will provide better insight into the design of more effective or selective homogeneous and heterogeneous catalysts. For example, vicinal diols are common motifs in biomass-derived materials, but their catalytic dehydrogenations have only been briefly studied. In this context, we have initiated an investigation focused on the homogeneous dehydrogenation of the vicinal diols as catalyzed by the Shvo catalyst1 (Ru2{(3,4-Tol2-2,5-Ph2C5O)2H}(µ-H)(CO)4, (1) and by Robinson's catalyst2 (Ru(CF3CO2)2(CO)(PPh3)2, (2). These dehydrogenations were operated in an open system in the acceptorless mode generating H2 quantitatively and in a closed system with the H2 acceptor diphenyl acetylene. Ongoing studies are attempting to determine the possible intermediates in the reaction. Parallel studies are evaluating the degradation of glycerol and glucose to H2, CO and CO2 using more extreme conditions and heterogenized ruthenium catalysts3.
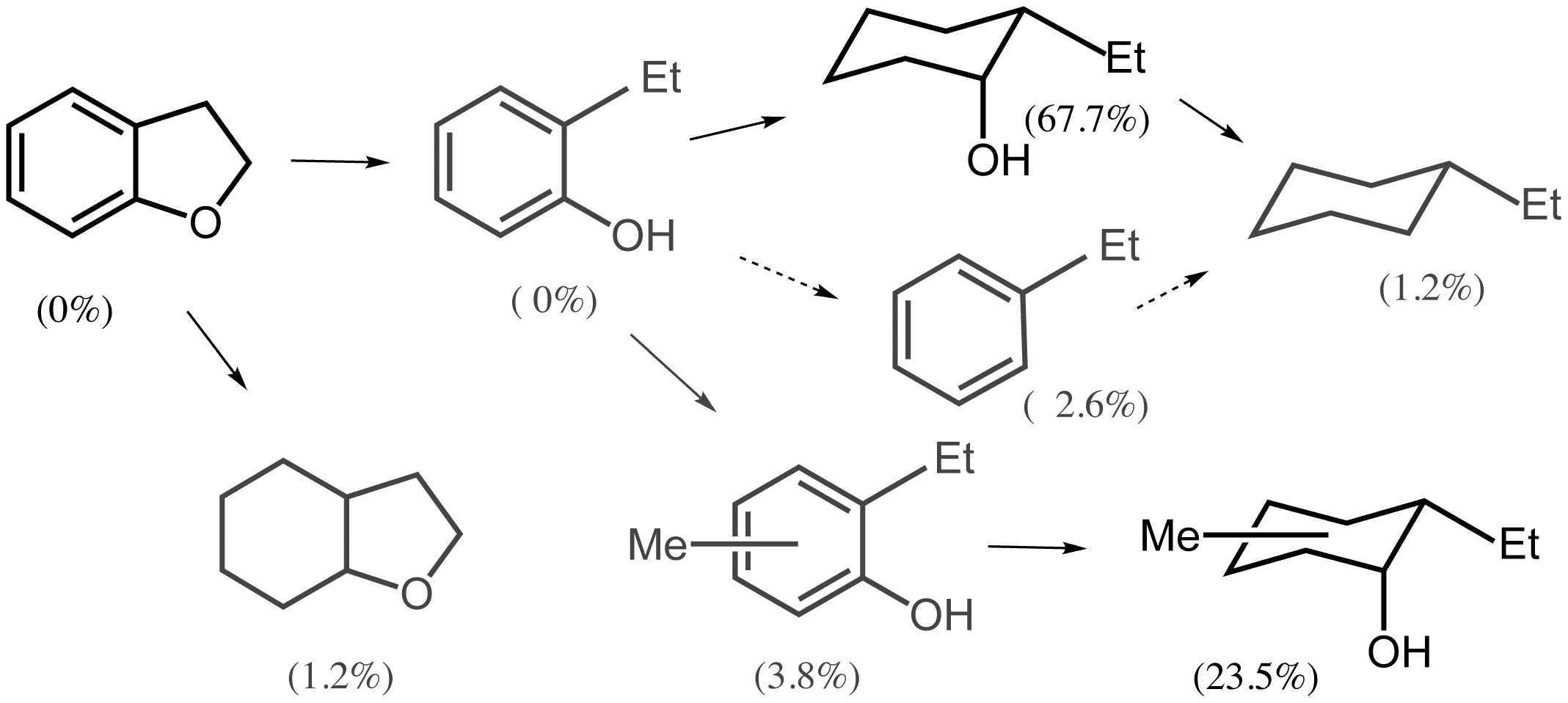
Much of our energy over the past year has been diverted to some very exciting developments regarding the hydrogen transfer from alcohols to lignin models, to lignin itself. and to lignocellulose composites. The long-term goal is to utilizing biomass derived H2 (or H2 equivalents) for the disassembly of lignin to more tractable monomeric fragments that might be used directly as liquid fuel components. Lignin is a heterogeneous biopolymer composed largely of 4-propylphenol derivatives coupled primarily as ethers but occasionally cross-linked with carbon-carbon bonds. We have found that porous metal oxides (PMOs) obtained by calcination of transition metal substituted Mg-Al hydrotalcite (HTC) precursors exhibit the properties of strong solid bases. For example, Fe-doped PMOs prepared from iron doped 3:1 Mg:Al HTCs are catalysts for transesterification reactions relevant to biodiesel production that outperform other catalysts typically used for this purpose.4 It was our premise that it would be possible to enhance the effectiveness of base-catalyzed hydrolysis of the phenyl ethers in lignins by introducing redox active transition metals into the formulation of the hydrotalcite precursors. Based on some literature precedents for the homogeneous alkali metal base degradation of lignin polymers in super-critical methanol (sc-MeOH), we rationalized that the solid bases might prove to be catalysts for this reaction. This premise did not prove successful, at least for the Fe-doped PMOs that were effective for biodiesel production. However, new HTC-derived PMO doped with Cu2+ are very effective catalysts for the transfer of H2 equivalents from sc-MeOH to the lignin model compound dihydrobenzofuran.5 The reaction appears to occur via sequential hydrogenolysis of the ether linkage and hydrogenation of the aryl group with methanol as the hydrogen source.
These studies have now been extended to the use of such PMO's for the reductive disassembly of organo-solv lignins largely to tractable monomeric aliphatic alcohols that may indeed have value as liquid fuel additives.6 Ongoing studies are directed toward evaluating such reactions for solid lignocellulose components such as sawdust and toward evaluating the possible use of biomass-derived alcohols to provide the H2 equivalents and with lignin models and for lignins themselves.
References:
(1) C. P. Casey, S. W. Singer, D. R. Powell, R. K. Hayashi, M. Kavana. J. Am. Chem. Soc. 2001, 123, 1090-1100.
(2) A. Dobson, S. D. Robinson. Inorg. Chem. 1977, 16, 137-142.
(3) T. D. Matson, G. Macala, P. C. Ford, studies in progress
(4) G. S. Macala, A. W. Robertson, C. L. Johnson, Z. B. Day, R. S. Lewis, M. G. White, A. V. Iretskii, P. C. Ford, Catal. Lets. 2008, 122, 205-209.
(5) G. S. Macala, T. D. Matson, C.L. Johnson, R. S. Lewis, A. V. Iretskii, P. C. Ford, ChemSusChem, 2009, 2, 215-217.
(6) K. Barta, T. D. Matson, M. L. Fettig, A. V. Iretskii, P.C. Ford, Manuscript in preparation.