Reports: AC3
45586-AC3 Tunable Control of Magnetic Properties in Transition Metal Indenyl Complexes
This research involves an investigation of the synthesis, structures, and magnetic properties of bis(indenyl)metal complexes that display adjustable changes in spin state. Ultimately we aim to incorporate the compounds into charge-transfer molecular magnets, and create new classes of magnetically ordered compounds. Some of these may display thermal hysteresis that would be a foundation for data storage applications.
The work of the first two years focused on bis(indenyl) complexes of chromium(II), which possess spin states (S = 1 or 2) that are strongly dependent on the orientation of the indenyl ligands; complexes with eclipsed ligands usually display thermally induced spin crossover. The origin of this behavior was traced with the aid of DFT calculations to symmetry restrictions on the mixing of the indenyl pi-orbitals with the chromium d orbitals; these limitations are relaxed under certain molecular conformations. We also completed an extensive survey of mono- and polymethylated bis(indenyl)chromium(II) complexes to determine the effect of donor substituents on magnetic behavior. In general, the presence of methyl groups favors low-spin complexes, but methyl groups on the six-membered benzo ring are somewhat more effective in this regard than are those on the five-membered ring.
Our most recent efforts have centered on manganese(II) indenyl chemistry. If complexes that display spin-crossover behavior can be integrated into magnetically ordered charge transfer salts, the resulting molecules could display thermally induced magnetic hysteresis. Some steps toward the goal had already been accomplished; the series of neutral bis(indenyl)Cr(II) complexes provided many examples of adjustable spin-crossover behavior, and we had previously prepared an indenyl-based charge transfer salt of Fe(III), [(1,2,3-Me3C9H4)2Fe][DCNQ] (DCNQ = 2,3-dicyanonaphtho-1,4-quinone), which was found to be magnetically ordered at low temperature. Incorporation of the chromium molecules into analogous salts was not an option, however, as the resulting d3 Cr(III) centers would not support spin crossover. Cationic Mn(III) complexes, which contain d4 metal centers, would be isoelectronic with neutral Cr(II) species, and might display comparable orientation-dependent spin-crossover behavior. Bis(indenyl)Mn(II) compounds would be the logical precursors to the Mn(III) cations.
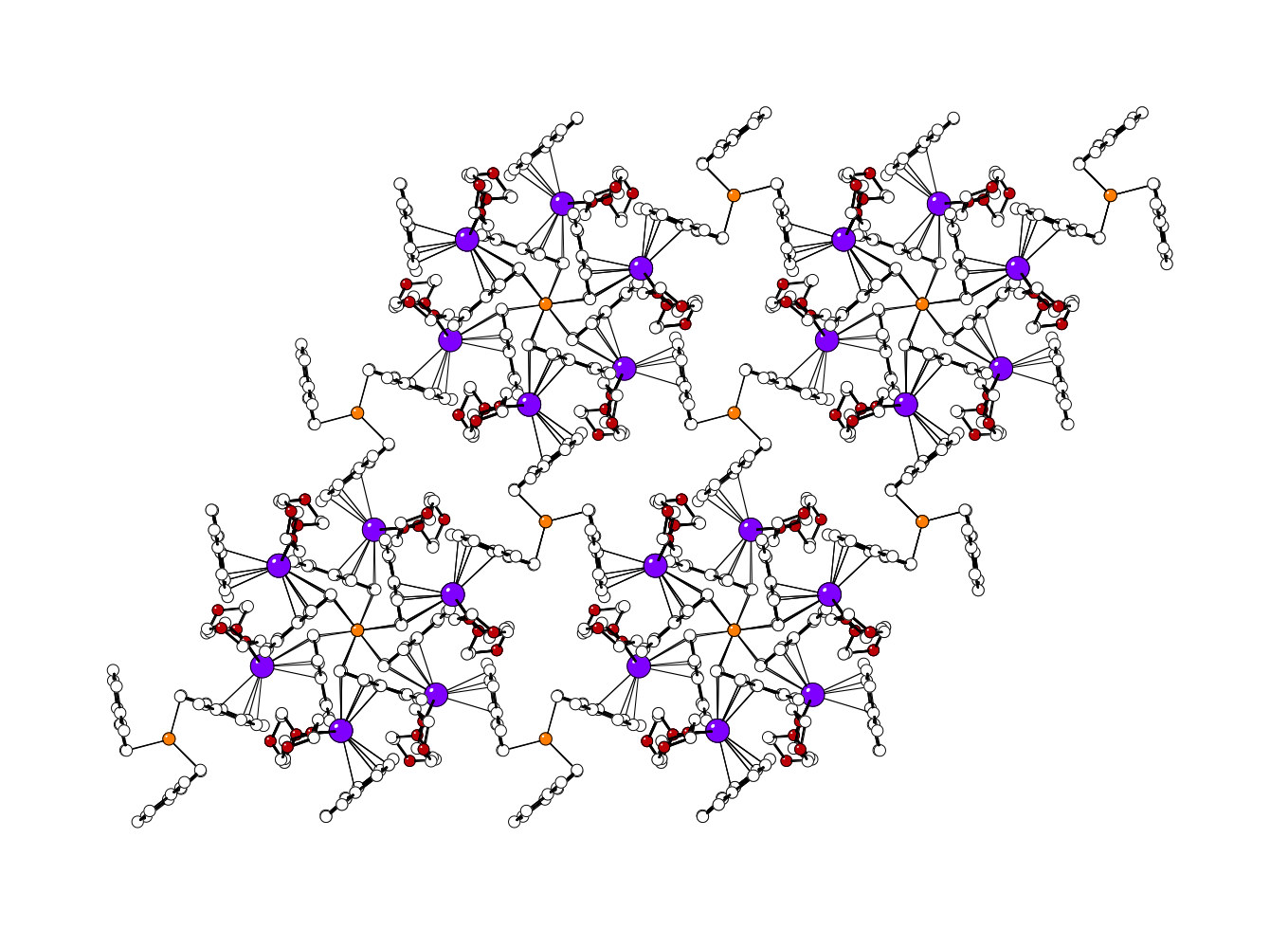
There is no (indenyl)Mn(II) chemistry described in the open literature, and the preparation of such compounds has been unusually challenging. Attempts to isolate the base-free complex (C9H7)2Mn have so far been unsuccessful, but we have obtained the THF-stabilized (C9H7)2Mn(thf)2, which has both η1 and η3-indenyl ligands. Various substituted derivatives, including [2-(SiMe3)2C9H5]2Mn and [1,3-(i-Pr)2C9H5]2Mn (both with staggered ligands) and [1,3-(SiMe3)2C9H5]2Mn (gauche ligands) have been synthesized. Several methylated derivatives have been prepared as well. When methyl groups are present on the benzo portion of the rings, specifically in the 4,7 positions, the corresponding complex is isolated as a cyclic octomer, {(4,7-Me2C9H5)2Mn}8, containing both bridging and terminal indenyl ligands. Additional extraction of the reaction mixture with 1,4-dioxane results in the isolation of the [K(dioxane)1.5][(Mn(Ind2Me-4,7)3] salt, in which each manganese atom is surrounded by a paddlewheel of three η2-bound 4,7-dimethylindenyl ligands. Cation–pi bonding to the potassium and the presence of coordinated dioxane molecules generates a layered structure for the salt (figure below, Mn centers in orange; K cations in purple).
The high reactivity of the molecules is indicated by the isolation of the dinuclear aryloxide complex (2-MeC9H6)2(μ-2-MeC9H6)Mn2(μ-BHT) (BHT = butylated hydroxytoluene) during the preparation of (2-MeC9H6)2Mn in THF stabilized with a small amount of BHT. Magnetic susceptibility measurements obtained to date indicate the presence of high-spin Mn(II) centers in all the complexes. Density functional theory calculations are consistent with highly flexible structures and low energies for rearrangement, in accord with a substantial degree of ionic bonding.
We have recently expanded the chemistry to include mono(indenyl) halide complexes, (Ind')MnX, which were originally obtained as unexpected by-products from the synthesis of the bis(indenyl) compounds. The halides can be obtained in high yield by adjusting the stoichiometry of the reaction mixtures, and X-ray crystal structures of the (Ind')MnX(thf) compounds (X = Cl, I) reveal that they are dimeric in the solid state. Magnetic data (SQUID measurements) on the chloro complex indicates that the metal centers are antiferromagnetically coupled.
The halide complexes display remarkable reactivity to oxygen at very low levels. Although solutions of (2,4,7-Me3C9H4)MnCl(thf) in rigorously degassed toluene are yellow at and below room temperature, they turn deep royal blue if cooled below room temperature under a nitrogen atmosphere. We initially attributed the color change to the formation of a dinitrogen complex (although this would be unprecedented for Mn(II)), but similar color changes were observed in the presence of other gases, including CO, H2, N2O, and even argon(!). It is now our understanding that the same species, a Mn superoxo complex, is formed in all these cases. It has a characteristic νOO stretch at 2119 cm–1. Evidently the trace levels of oxygen present in even UHP grades of gases (ca. 2 ppm) are enough to initiate superoxide formation. Manganese superoxo complexes are known in other contexts, but the ease with which the indenyl complexes generate such species is uncommon, even if not completely unprecedented. As the superoxo complex cannot be synthesized in THF solution, dissociation of coordinated THF from the manganese center of the indenyl halide is evidently one of the first steps in its formation. We are continuing our study of the superoxo chemistry, which may lead to the development of new magnetically useful materials.
This research has moved the PI's research in new directions in synthesis and structural characterization. It has also led to increasing use of computer modeling in the interpretation of experimental results. Both the PI and the graduate students involved in the project have given several presentations on the research at regional and national ACS meetings.