Reports: G5
47747-G5 Immobilization of Enzymes on Nanoparticles for Catalysis in Non-aqueous Media
Introduction
Enzymes are highly functional biomolecular catalysts that have extremely high specificity and very fast reaction rates. The properties and structure of an enzyme can be to a large extent affected by its environment. Reaction medium, salt content, temperature and many other variables play important roles in the activity of an enzyme. Enzymes have been shown to have activity in non-aqueous media, though considerably lower than the activity in native aqueous conditions. The reason for such decreased activity is enzyme unfolding in the presence of a non-aqueous solvent. It has also been shown that enzymes attached to nanoparticles are stabilized in conditions that would normally negatively affect the activity of an enzyme. We hypothesized that activity in non-aqueous media would be higher for enzyme attached to nanoparticles than for free enzyme. One application of such a system would be in the purification of fossil fuels. Our ultimate goal is to use enzymes stabilized on nanoparticles in organic solvents to remove sulfur compounds by oxidative desulfurization.
Materials and Methods
Chloroperoxidase is the enzyme of choice for these experiments because it has been shown to exhibit oxidative desulfurization in non-aqueous media. Latex nanoparticles with a diameter of ~40 nm surface modified with chloromethyl reactive groups were used in the experiments. Attachment to nanoparticles was performed in phosphate buffer saline overnight at room temperature. All nanoparticles were separated using centrifugation at 12,000 g for 2 h. A BCA protein assay (Pierce) was used to quantitatively evaluate enzyme attachment to nanoparticles. The nanoparticle conjugates were then lyophilized to remove water. Enzyme desorption from the nanoparticle surface was studied using fluorescently labeled chloroperoxidase. Enzyme activity assays were performed both in aqueous and non-aqueous media. Aqueous activity assays were performed in deionized water with 20 mM N,N,N',N'-tetramethyl-p-phenylenediamine (TMPD) and 2 mM Hydrogen Peroxide as the enzyme's oxygen source. Activity was measured by following the oxidation of TMPD and monitoring the change in absorbance spectrophotometrically at 562 nm. Non-aqueous activity assays were performed in 99.5% ethanol. Activity in ethanol was measured by the same method that was employed for aqueous media so that the effect of immobilization on nanoparticles could be determined. Free lyophilized enzyme was used as control; concentrations of free and immobilized enzyme were kept identical in all kinetic experiments.
Results and Discussion
Amine groups of chloroperoxidase react with chloromethyl groups on the nanoparticles resulting in covalent attachement of the enzyme to nanoparticles. After overnight incubation of chloroperoxidase to nanoparticles, 87% of the enzyme was bound to the nanoparticles. Addition of the surfactant (Tween 20) to remove non-specifically adsorbed protein reduced binding yield to 71%. Results from the leaching study revealed that there was a loss of ~4% of the immobilized enzyme over the 72 h incubation in an aqueous buffer.
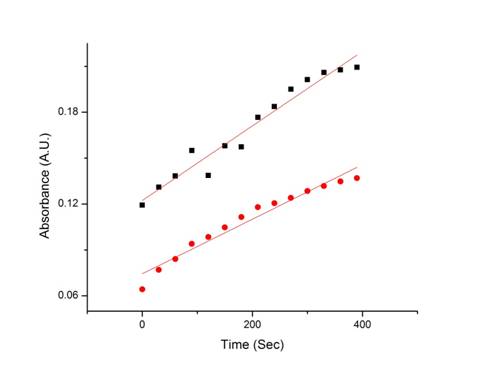
Activity assays were performed on the enzyme-nanoparticle conjugates as well as free enzyme to see the effect of immobilization on nanoparticles on enzyme activity. We observed enzyme activity in both the free enzyme and the enzyme-nanoparticle conjugates in 99.5% ethanol. A comparison of enzyme activity was performed to determine the effect of immobilization. Results indicate a 33% higher initial rate in the enzyme-nanoparticle sample when compared to free enzyme (Figure 1). This finding supports the hypothesis that the enzyme will be stabilized when attached to the nanoparticle. We also expect to find that the half-life of the enzyme increases in the conjugate as compared to the free enzyme. This favorable outcome indicates that this method of immobilization may well result in the desired effects of extended activity and enzyme stability in harsh environments.
Conclusions and future work
The results obtained here suggest that our method of enzyme attachment to nanoparticles could be an effective method of immobilization. We also demonstrated that reaction in non-aqueous media occurs for both free enzyme and enzyme immobilized on nanoparticles. As expected, the reaction rate of enzyme in non-aqueous solvents was lower than in aqueous media. However, reaction rate for the enzyme immobilized on nanoparticles in non-aqueous solvent was higher than that for free enzyme. This data is encouraging, but additional steps are necessary to optimize the reaction conditions in non-aqueous media.
During the second year of the project we will perform additional experiments in ethanol to verify reproducibility of the observed effect. We will also study effect of aging time on enzyme activity in ethanol for both immobilized and free enzyme and compare their long-term storage stability. Finally, we plan to perform a pilot study on oxidation of thiophene, monitored by HPLC, by chloroperoxidase-nanoparticle conjugates suspended in diesel fuel.
Figure 1: Activity of NP-CPO conjugates is compared to
that of the free enzyme. Slope for NP-CPO conjugates is 33% higher than the
slope for free enzyme. Free enzyme (1.8E-4) Conjugates (2.4E-4)