Reports: G10
48108-G10 In Situ Electron Microscopy Studies of the Kirkendall Effect in Small Clusters
During the first year of this program funded by the ACS PRF starter grant, we carried out the following research, broadly focused on in situ as well as ex situ transmission electron microscopy (TEM) characterization of catalytically-active nanoparticles. We investigated the 1) chemical and thermal stabilities of C-coated titania nanocrystals, 2) thermal stability of K-sodalite particles, and 3) structural and morphological characterization of transition-metal (TM) oxide and Au nanocrystals.
1) In situ TEM studies of chemical and thermal stabilities of C-coated titania nanocrystals: Titanium carbide (TiC) and other TM carbides are promising for applications as heterogeneous catalysts, as structural reinforcements, and as refractory coatings. TM carbide powders are commonly produced via carbothermal reduction of TiO2 at elevated temperatures (>1200 ºC). This reduction reaction is suggested to occur via successive formation of lower oxides of titanium along with the emission of CO and CO2 gases. However, details of the reaction kinetics, which control the final particle size, shape, and crystal structure are largely unknown.
 In order to
understand the mechanisms controlling the carbothermal reduction process, we
used in situ TEM and followed the high-temperature dynamics of C-coated
titania particles. These samples are prepared by pyrolysis of propylene at ~
600 oC, which resulted in a uniform coating of pyrolytic carbon
shell (thickness ~2-5 nm) around individual oxide particles. In situ lattice-resolution
TEM images are acquired at video rate (15 frames/s) while heating the particles
in vacuum up to 1000 oC for times up to 5 h. Energy dispersive X-ray
spectra (EDX) are obtained at room temperature from the samples before and
after the annealing experiments. We find several interesting phenomena: 1) graphitization
of carbon preferentially on the lowest-energy planes of TiO2; 2)
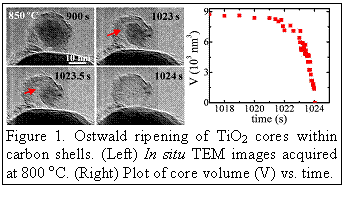
shrinking and eventual disappearance of the oxide cores while being
encapsulated by carbon, resulting in the formation of hollow-core graphene
shell structures (see Fig. 1); 3) reduction of TiO2 to lower oxides.
These studies provide kinetic information and atomic-scale insights into the
early stage carbothermal reduction process leading to the synthesis of TiC
particles.
In order to
understand the mechanisms controlling the carbothermal reduction process, we
used in situ TEM and followed the high-temperature dynamics of C-coated
titania particles. These samples are prepared by pyrolysis of propylene at ~
600 oC, which resulted in a uniform coating of pyrolytic carbon
shell (thickness ~2-5 nm) around individual oxide particles. In situ lattice-resolution
TEM images are acquired at video rate (15 frames/s) while heating the particles
in vacuum up to 1000 oC for times up to 5 h. Energy dispersive X-ray
spectra (EDX) are obtained at room temperature from the samples before and
after the annealing experiments. We find several interesting phenomena: 1) graphitization
of carbon preferentially on the lowest-energy planes of TiO2; 2)
shrinking and eventual disappearance of the oxide cores while being
encapsulated by carbon, resulting in the formation of hollow-core graphene
shell structures (see Fig. 1); 3) reduction of TiO2 to lower oxides.
These studies provide kinetic information and atomic-scale insights into the
early stage carbothermal reduction process leading to the synthesis of TiC
particles.
2) In situ TEM investigation of the thermal stability of K-sodalite (K-SOD) particles: K-SOD is a promising material for removing diesel soot in automobiles. Recent studies have shown that the catalytic activity of K-SOD improves upon annealing at high temperatures (~ 800 oC). However, the reasons for this behavior are not clearly understood. Therefore, we investigated the temperature-dependent morphological, structural, and compositional evolution in K-SOD powders. To this purpose, we used in situ TEM and observed individual K-SOD particles during annealing at 600, 800 and 1000 oC. During heating, we observe a decrease in size, crystal structure changed from that of sodalite to nepheline, and Na-rich compounds appeared on the surface. The transitions started at temperatures between 600 and 700 oC. Further studies are required to fully understand these transformations.
3) Structural and morphological characterization of transition-metal oxide and Au nanocrystals: Metal and metal-oxide nano-structures have potential for applications in catalysis, separations, microelectronics and medicine. To-date, these nanostructures have been synthesized using a variety of wet-chemical methods. Recently, our colleague Prof. Richards at the Colorado School of Mines and co-workers developed a simple and template-free method for producing hierarchically self-assembled architectures with tailored chemical compositions and controlled morphologies. Here, we report the structural and morphological characterization of Au, Cr2O3, and Co3O4 powders provided by Prof. Richards and his graduate student Ms. Lifang Chen.
 Nano Au: We used scanning
electron microscopy (SEM) and TEM to characterize Au nanocrystals synthesized using HAuCl4·3H2O as the precursor with
glucose as the reducing reagent and sodium dodecyl sulphate as the directing
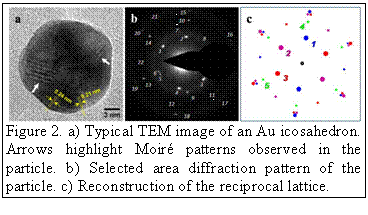
agent at room temperature. SEM and TEM images reveal that the gold icosahedra of
controllable sizes (30 - 250 nm) are obtained. These icosahedra
are composed of multiple rotational twins that are likely due to assembly of
tetrahedral units (see Fig. 2). These nanocrystals exhibit high activity
in the reduction of p-nitrophenol by sodium borohydride.
Nano Au: We used scanning
electron microscopy (SEM) and TEM to characterize Au nanocrystals synthesized using HAuCl4·3H2O as the precursor with
glucose as the reducing reagent and sodium dodecyl sulphate as the directing
agent at room temperature. SEM and TEM images reveal that the gold icosahedra of
controllable sizes (30 - 250 nm) are obtained. These icosahedra
are composed of multiple rotational twins that are likely due to assembly of
tetrahedral units (see Fig. 2). These nanocrystals exhibit high activity
in the reduction of p-nitrophenol by sodium borohydride.
 Co3O4: We examined,
using SEM and TEM, the morphologies and microstructures of cobalt oxide powders
synthesized via template-free approach and after calcinations at 350 oC
for 3 h and the other at 500 oC for 2 h. In addition, we also
carried out in situ TEM observations of the calcination process. SEM
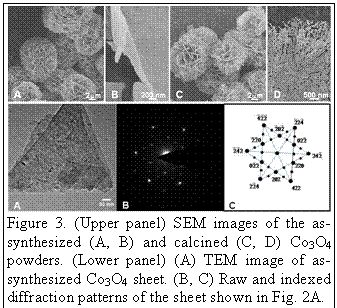
images of the as-synthesized powders show that the powders are uniform in size,
fully dispersed, and appear spherical. At higher magnifications, we find that
these microspheres are composed of nanoscale sheets. After calcination at 500 oC
for 2 hours, the overall morphology, the size and the crystal structure of the
spheres did not change significantly, however, the sheets are now composed of facetted
pores with edges oriented mostly perpendicular to <112>. TEM and SAED
analyses indicate that the sheets are single-crystalline, exhibit cubic Fd3m
(227) (a=8.084 Å) spinel structure, and are oriented perpendicular to
<111>.
Co3O4: We examined,
using SEM and TEM, the morphologies and microstructures of cobalt oxide powders
synthesized via template-free approach and after calcinations at 350 oC
for 3 h and the other at 500 oC for 2 h. In addition, we also
carried out in situ TEM observations of the calcination process. SEM
images of the as-synthesized powders show that the powders are uniform in size,
fully dispersed, and appear spherical. At higher magnifications, we find that
these microspheres are composed of nanoscale sheets. After calcination at 500 oC
for 2 hours, the overall morphology, the size and the crystal structure of the
spheres did not change significantly, however, the sheets are now composed of facetted
pores with edges oriented mostly perpendicular to <112>. TEM and SAED
analyses indicate that the sheets are single-crystalline, exhibit cubic Fd3m
(227) (a=8.084 Å) spinel structure, and are oriented perpendicular to
<111>.
Cr2O3: Using chromium oxide as a model system, we constructed a morphological "phase diagram" for chromium oxide spheres that shows the evolution of nanoparticle size and surface roughness as a function of precursor and urea concentrations. It is notable that these chromium oxide spheres show an exceptional ability to remove azo-dye pollutant in water treatment. Using SEM and TEM, we determined their morphologies and crystal structure. We find that the as-synthesized Cr2O3 powders are spherical, exhibit porosity, and are composed of polycrystalline (corundum structure) nanoscale particulates. Additional details are presented in the article published in ACS Applied Materials & Interfaces.




