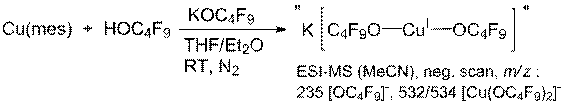Reports: AC3
48022-AC3 Late Transition Metal Complexes with Fluorinated Alkoxide Ligands for C-H Activation
We proposed the syntheses and reactivity studies of a new family of late first row transition metal complexes having oxygen donor ligands in a range of low, medium and high oxidation states with fluorinated alkoxide oxygen donor ligands. Our original goals were (i) initial preparation of new transition metal complexes with convenient oxidation states, (ii) subsequent preparation of low and high oxidation species without additional ligands, (iii) preparation of high oxidation state complexes via atom transfer reactions, (iv) placement of oxygen donor ligands such as OC4F9, OCH(CF3)2 and related species in a traditional ligand field series, and (v) to survey these new reactive systems for those that may be developed into catalytic C-H oxidation systems under mild conditions. We report here in that we have accomplished goals (i) and (iv), made progress on (ii) and stand well-prepared to focus on the remaining objectives
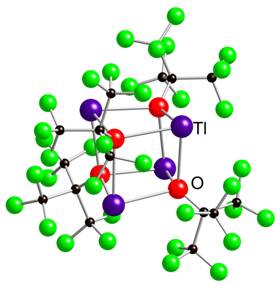
The alcohols HOC(CF3)3 (= HORF) and HOC(CF3)2Ph have been the most investigated in this project to date. Both potassium and thallium alkoxide derivatives have been prepared as important reagents and their characterization, including structural studies has been completed. Shown below are the cubane structures of {Tl(ORF)}4 and {K(ORF)}4. The lack of solvent coordination for thallium is not unusual given the Tl(I) oxidation state and potential for a stereochemically active lone pair. In the K(I) derivative, there are only one and half molecules of toluene present per cubane and both {M(ORF)}4 structures show significant interactions between the fluoride atoms and the metal centers. In the potassium case, a chain of K F linkages extends throughout the solid state.
Homoleptic Fluorinated Alkoxide Complexes
Using the procedures previously developed in our laboratory for syntheses of Cu(II), Ni(II), Co(II), Fe(II) and Fe(III) fluorinated homoleptic aryloxide compounds, we have succeeded in the synthesis of two new families of fluorinated homoleptic alkoxide compounds, namely [M(ORF)3]1- (M=Fe,Co,Cu,Zn) and [M(ORF)4]2- (M=Co, Ni). All of these compounds have been prepared as {K(18-crown-6)}+ salts and all except the [Cu(ORF)3]1- derivative have been structurally characterized. The [MX3]- anions have trigonal planar geometry at the metal centers and the four-coordinate species are tetrahedral. This work was published earlier this year.
One of the goals of the research was to place these fluorinated alkoxide ligands in the context of the conventional ligand field series. The UV-vis-near-IR spectra of the [Ni(OAr)4]2- and [Ni(OR)4]2- compounds as well as that of other (pseudo)halogen [NiX4]2- are listed in Table 1. The data were ananlyzed according to the method of Lever to obtain values for the Racah parameter B and the ligand field parameter Dq. All four spectra of the Ni complexes with fluorinated ligands show significantly blue shifted spectra, compared to [NiCl4]2-, indicating a stronger ligand field. The strongly electron-withdrawing and reduced p-basic character of the ligands result in a large ligand field splitting as described by Dq. The large Racah parameter B for the alkoxide derivative indicates strong interelectronic repulsion and primarily s-donor character from the ligand. Based on these data we write the following abbreviated spectrochemical series:
Br < Cl < NCO << OAr' ~ OArF < ORF
Table 1. Electronic Spectral Data and Ligand Field Parameters
|
[NiX4]2-
|
E(n3) (cm-1)
|
E(n2) cm-1
|
B (cm-1)
|
Dq (cm-1)
|
|
[Ni(NCO)4]2-
|
16,200
|
9460
|
511
|
311
|
|
[NiCl4]2-
|
14760
|
7470
|
405
|
206
|
|
[NiBr4]2-
|
13,320
|
6995
|
379
|
201
|
|
[Ni(OArF)4]2-, 2
|
16,660
|
9290
|
877
|
502
|
|
[Ni(OAr′)4]2-, 3
|
16,820
|
10,000
|
867
|
540
|
|
[Ni(OArF)4]2-, 4
|
16,000
|
10,400
|
800
|
560
|
|
[Ni(ORF)4]2-, 5
|
19,300
|
7840
|
1096
|
427
|
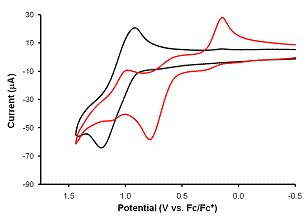
Electrochemical
studies on the pair of compounds [Co(ORF)4]2-
and [Co(ORF)3]- have yielded interesting data
about the stability of these compounds in solution as well a the energies of
the Co(III)/Co(II) reduction potentials. UV-vis studies (not shown) indicated that the 4-coordinate
species dissociated one ligand to form the three coordinate one. Excess alkoxide regenerated the tetrahedral
anion. The black trace in the cyclic voltammogram
below is that for the [Co(ORF)3]- species
which has a Co(III)/Co(II) midpoint potential ~ 1.3 V (vs Cp2Fe+/Cp2Fe)
and the red trace is for [Co(ORF)4]2-
in the presence of excess alkoxide in which a midpoint potential shift to ~ 0.9
V is apparent. It is therefore easier to
stabilize the higher oxidation state with the higher coordination number.
DFT
Computational Studies
We
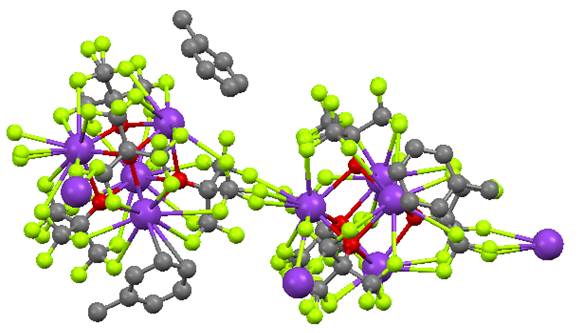
have also extended the fluorinated ligands to Cu(I) in the synthesis of a pair
of two-coordinate linear [Cu(OR)2]- species. Shown below is the preparation of OC(CF3)3
derivative and the same synthesis has been achieved for the OCPh(CF3)2
alkoxide. Both compounds have been
structurally characterized and also react with O2 at low
temperatures, but the spectra of those species are quite distinct. Notably, one of the ligands contains C-H
bonds, and the other does not.
Future
Directions
Currently
we are pursuing higher and lower oxidation states of the Fe(II), Co(II), and
Ni(II) compounds, as well as the synthesis of neutral M(II) [M(ORF)2]2
compounds which we predict will be dimeric for these metals. A great deal of emphasis is being placed on
the reactivity in the Cu(I)/O2 systems where we currently have the
highest potential for discovering C-H reactivity. Manometry experiments are underway as well as reactivity
studies with a variety of substrates.
 A comparative study of the electronic
structures of several high spin [CoX3]- compounds was
completed, and the simplified diagram with the a-spin
orbitals is shown below. The key results
are that the fluorination greatly lowers the HOMO compared to other related
systems but the p-donor ability of the ligand is
reduced as shown by the relatively lower energies of the (xz,yz) pair vs the
amide ligand. The latter feature was
demonstrated experimentally in the ligand field studies above.
A comparative study of the electronic
structures of several high spin [CoX3]- compounds was
completed, and the simplified diagram with the a-spin
orbitals is shown below. The key results
are that the fluorination greatly lowers the HOMO compared to other related
systems but the p-donor ability of the ligand is
reduced as shown by the relatively lower energies of the (xz,yz) pair vs the
amide ligand. The latter feature was
demonstrated experimentally in the ligand field studies above.