Reports: AC1
47942-AC1 Synthesis and Application of C2-Chiral Phosphinines to Asymmetric Catalysis
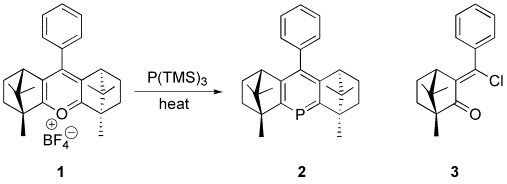
One area of research in my laboratories is the development of C2-asymmetric phosphinines, also known as phosphabenzenes. Phosphinines are a relatively new type of transition metal ligand, their use in catalysis dating to only 1996. Rhodium-phosphinine complexes exhibit high reactivity as hydroformylation catalysts, yet only five chiral variants have been reported and none that possess the desirable C2 symmetry. We have developed the synthesis of the first C2-asymmetric phosphinine (2) from (+)-camphor by a five-step procedure that proceeds via bis-camphorpyrylium 1, the first C2-chiral (and only the third asymmetric) pyrylium of any type to be reported. Phosphinine 2 is a crystalline, air-stable and chromatographable material that strongly coordinates the late transition metals. The efficacy of this ligand in asymmetric catalysis was tested in palladium-catalyzed hydrosilylation/oxidation of styrene, yielding 1-phenyl-1-ethanol in 27% ee. Based on the X-ray crystal structure, we attribute this poor performance to two factors: (a) the limited ability of the bridgehead methyl groups to encroach on the metal's coordination sphere, and (b) the shallow angle (18°) at which these methyl groups deviate from the plane of the phosphinine ring. Other monodentate phosphinines, as well as bidentate ligands, are being pursued. In addition, we are taking advantage of an intermediate (3) in the synthesis of 2 that may allow easy access to a variety of C1 ligands.